Titration First Endpoint . Using a ph probe and using a colour indicator. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: during the process, two important stages known as endpoint and equivalence point are reached. another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A point of equivalence in a. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. You will also learn about equivalence points. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.
from www.numerade.com
Using a ph probe and using a colour indicator. A point of equivalence in a. during the process, two important stages known as endpoint and equivalence point are reached. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. You will also learn about equivalence points. another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. for a ph titration (between an acid and a base), there are two common ways to find the endpoint:
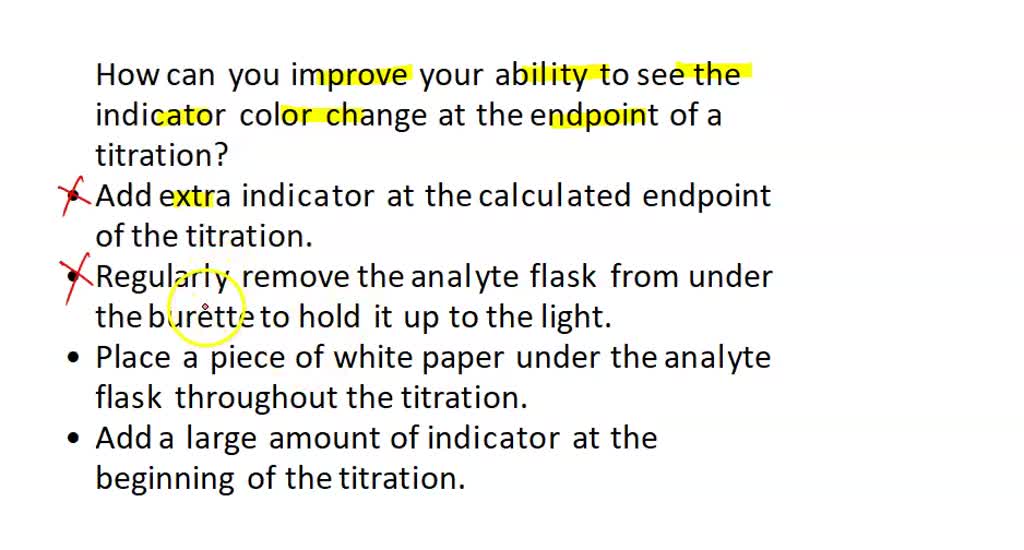
SOLVED How can you improve your ability to see the indicator color
Titration First Endpoint for a ph titration (between an acid and a base), there are two common ways to find the endpoint: for a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Using a ph probe and using a colour indicator. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. during the process, two important stages known as endpoint and equivalence point are reached. A point of equivalence in a. another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. You will also learn about equivalence points.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration First Endpoint Using a ph probe and using a colour indicator. You will also learn about equivalence points. during the process, two important stages known as endpoint and equivalence point are reached. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: another method for locating the end point is. Titration First Endpoint.
From dxolxlskr.blob.core.windows.net
Why Use Titration To Find Concentration at Rodney Ruggerio blog Titration First Endpoint a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. Using a ph probe and using a colour indicator. during the process, two important stages known as endpoint and equivalence point are reached. for a ph titration (between an acid and a base), there are two common ways to. Titration First Endpoint.
From pubs.sciepub.com
Figure 9. First derivative plot of the titration of weak acid (CH3COOH Titration First Endpoint for a ph titration (between an acid and a base), there are two common ways to find the endpoint: You will also learn about equivalence points. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration is a volumetric technique in which a solution of one reactant. Titration First Endpoint.
From www.numerade.com
SOLVED How can you improve your ability to see the indicator color Titration First Endpoint a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. A point of equivalence in a. You will also learn about equivalence points. for a ph titration (between an acid and a base), there are two common ways to find. Titration First Endpoint.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration First Endpoint another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. Using a ph probe and using a colour indicator. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. during the process, two important stages known as. Titration First Endpoint.
From quizpseudology.z21.web.core.windows.net
How To Find Equivalence Point Volume Titration First Endpoint another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Using a ph probe and using a colour. Titration First Endpoint.
From www.chegg.com
Titration Molarity of NaOH 0.1004 M 1st endpoint Titration First Endpoint Using a ph probe and using a colour indicator. another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. You will also learn about equivalence points. during the process, two important stages known as endpoint and equivalence point are reached. a titration is a technique. Titration First Endpoint.
From www.animalia-life.club
Endpoint Titration Titration First Endpoint during the process, two important stages known as endpoint and equivalence point are reached. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. Using a ph probe and using a. Titration First Endpoint.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 Titration First Endpoint for a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. A point of equivalence in a. Using a ph probe and using a colour indicator. a titration is a volumetric. Titration First Endpoint.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog Titration First Endpoint Using a ph probe and using a colour indicator. You will also learn about equivalence points. A point of equivalence in a. another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. in this tutorial, you will learn about titration curves, titration analysis and the steps. Titration First Endpoint.
From www.animalia-life.club
Endpoint Titration Titration First Endpoint a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Using a ph probe and using a colour indicator. during the process, two important stages known as endpoint and equivalence point are reached. in this tutorial, you will learn. Titration First Endpoint.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration First Endpoint Using a ph probe and using a colour indicator. another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. a titration is a technique used in chemistry. Titration First Endpoint.
From www.maieutapedia.org
Stoichiometry and Titration Maieuta Pedia Titration First Endpoint another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. You will also learn about equivalence points. Using a ph probe and using a colour indicator. for. Titration First Endpoint.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID3970559 Titration First Endpoint a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will. Titration First Endpoint.
From letitsnowglobe.co.uk
Titration procedure pdf Titration First Endpoint during the process, two important stages known as endpoint and equivalence point are reached. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. A point of equivalence in a.. Titration First Endpoint.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Titration First Endpoint during the process, two important stages known as endpoint and equivalence point are reached. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: another method for locating the. Titration First Endpoint.
From mungfali.com
Endpoint Titration Curve Titration First Endpoint You will also learn about equivalence points. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Using a ph probe and using a colour indicator. during the process, two important stages known as endpoint and equivalence point are reached.. Titration First Endpoint.
From pubs.sciepub.com
Figure 16. Second derivative plot of the titration of chloride ion (0 Titration First Endpoint in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed. Titration First Endpoint.
From dxoyufult.blob.core.windows.net
Titration Analysis Cation at Sean Eubanks blog Titration First Endpoint Using a ph probe and using a colour indicator. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. during. Titration First Endpoint.
From www.youtube.com
Excel The Solver for polynomials and equilibrium conc. 1st and 2nd Titration First Endpoint You will also learn about equivalence points. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. Using a ph probe and using a colour indicator. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. A point of equivalence in a.. Titration First Endpoint.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration First Endpoint You will also learn about equivalence points. A point of equivalence in a. during the process, two important stages known as endpoint and equivalence point are reached. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. in this. Titration First Endpoint.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration First Endpoint during the process, two important stages known as endpoint and equivalence point are reached. another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. A point of equivalence in a. for a ph titration (between an acid and a base), there are two common ways. Titration First Endpoint.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titration First Endpoint for a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. another method for locating the end point is to plot. Titration First Endpoint.
From www.youtube.com
Back Titration Example YouTube Titration First Endpoint A point of equivalence in a. You will also learn about equivalence points. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Using a ph probe and using a colour indicator. in this tutorial, you will learn about titration. Titration First Endpoint.
From dxobegonq.blob.core.windows.net
Titration Curve Labels at Ok Moore blog Titration First Endpoint You will also learn about equivalence points. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. for a ph titration (between an acid and a base), there are two common. Titration First Endpoint.
From ar.inspiredpencil.com
Titration Curve Endpoint Titration First Endpoint another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. Using a ph probe and using a colour indicator. in this tutorial, you will learn about titration. Titration First Endpoint.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Titration First Endpoint a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. during the process, two important stages known as endpoint and equivalence point are reached. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. for a ph titration (between an. Titration First Endpoint.
From mavink.com
Titration Labeled Titration First Endpoint a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Using a ph probe and using a colour indicator. A point of equivalence in a. during the process, two important stages known as endpoint and equivalence point are reached. . Titration First Endpoint.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Titration First Endpoint during the process, two important stages known as endpoint and equivalence point are reached. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Titration First Endpoint.
From theedge.com.hk
Chemistry How To Titration The Edge Titration First Endpoint another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Using a ph probe and using a colour indicator. during the process, two important stages known as. Titration First Endpoint.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration First Endpoint a titration is a technique used in chemistry to help determine the concentration of a reactant mixed within. You will also learn about equivalence points. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: A point of equivalence in a. during the process, two important stages known. Titration First Endpoint.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID737892 Titration First Endpoint another method for locating the end point is to plot the titration curve’s first derivative, which gives the titration curve’s slope at. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Using a ph probe and using a colour. Titration First Endpoint.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration First Endpoint a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Using a ph probe and using a colour indicator. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: You will. Titration First Endpoint.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration First Endpoint You will also learn about equivalence points. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Using a ph probe and using a colour indicator. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration is a. Titration First Endpoint.
From pubs.sciepub.com
Figure 6. First derivative plot of the titration of strong acid (HCl= 0 Titration First Endpoint during the process, two important stages known as endpoint and equivalence point are reached. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Titration First Endpoint.