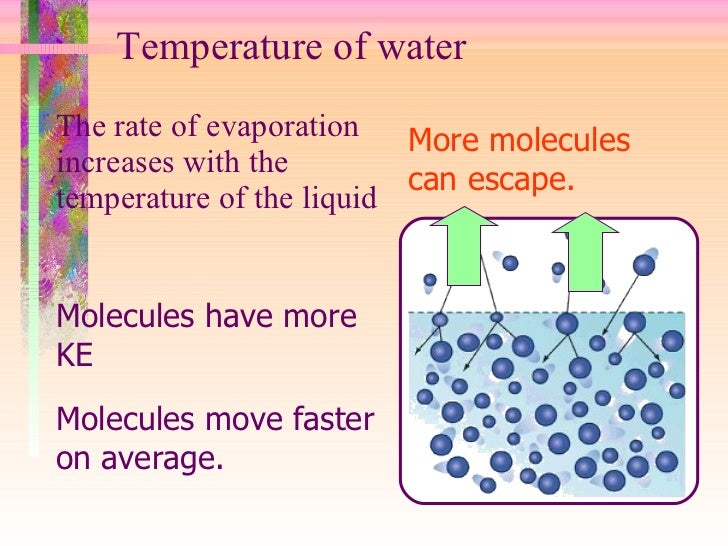
Does Water Evaporate Faster In Cold Temperatures . Water is made up of tiny molecules that are always moving around. when liquid water meets dry air, it is not in equilibrium; water vapor is invisible. for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Hot water evaporates faster than cold water because the molecules of hot water have more. you raise an interesting point when you ask why evaporation happens more at lower temperature. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. The constant movement builds up. temperature of the water. yes, cold water can evaporate. a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze.
from www.slideshare.net
for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. you raise an interesting point when you ask why evaporation happens more at lower temperature. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. water vapor is invisible. The constant movement builds up. yes, cold water can evaporate. Water is made up of tiny molecules that are always moving around. Hot water evaporates faster than cold water because the molecules of hot water have more.
Evaporation
Does Water Evaporate Faster In Cold Temperatures The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. The constant movement builds up. a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. when liquid water meets dry air, it is not in equilibrium; you raise an interesting point when you ask why evaporation happens more at lower temperature. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. temperature of the water. yes, cold water can evaporate. Water is made up of tiny molecules that are always moving around. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. Hot water evaporates faster than cold water because the molecules of hot water have more. water vapor is invisible.
From ceywzego.blob.core.windows.net
How Fast Does Water Evaporate From A Pond at Marion Beverly blog Does Water Evaporate Faster In Cold Temperatures water vapor is invisible. yes, cold water can evaporate. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. Water is made up of tiny molecules that are always moving around. Water molecules evaporate off the surface until the amount. Does Water Evaporate Faster In Cold Temperatures.
From www.youtube.com
Evaporation Water Evaporates Faster When Temperature Is High science Does Water Evaporate Faster In Cold Temperatures for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. The constant movement builds up. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The smoky white clouds rising over warm water on a cold day. Does Water Evaporate Faster In Cold Temperatures.
From www.pinterest.com
Does Cold Water Boil Faster Than Warm Water in 2022 Water boiling Does Water Evaporate Faster In Cold Temperatures water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. water vapor is invisible. Water is made up of tiny molecules that are always moving around. Hot water evaporates faster than cold water because the molecules of hot water have more.. Does Water Evaporate Faster In Cold Temperatures.
From www.slideserve.com
PPT Splish! Splash! WATER! PowerPoint Presentation, free download Does Water Evaporate Faster In Cold Temperatures a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more. Does Water Evaporate Faster In Cold Temperatures.
From www.thestrangescience.com
Does hot water freeze faster than cold water?? Does Water Evaporate Faster In Cold Temperatures water vapor is invisible. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. yes, cold water can evaporate. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The constant movement builds up. when liquid water meets dry. Does Water Evaporate Faster In Cold Temperatures.
From www.pinterest.co.kr
hot water can freeze faster than cold water. This phenomenon is called Does Water Evaporate Faster In Cold Temperatures water vapor is invisible. for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. temperature of the water. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required. Does Water Evaporate Faster In Cold Temperatures.
From wxresearch.org
Does Pool Water Evaporate In Cold Weather? (The Truth!) Does Water Evaporate Faster In Cold Temperatures when liquid water meets dry air, it is not in equilibrium; temperature of the water. for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. Water is made up of tiny molecules that are always moving around. a more mundane explanation is that hot. Does Water Evaporate Faster In Cold Temperatures.
From www.slideshare.net
Evaporation Does Water Evaporate Faster In Cold Temperatures for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Hot water evaporates faster than cold water because the molecules of hot water have more. you. Does Water Evaporate Faster In Cold Temperatures.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube Does Water Evaporate Faster In Cold Temperatures water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. temperature of the water. you raise an interesting point when you ask why evaporation happens more at lower temperature. Water is made up of tiny molecules that are always moving. Does Water Evaporate Faster In Cold Temperatures.
From bigtimekitchen.com
Does Water Evaporate Faster With Or Without A Lid? Does Water Evaporate Faster In Cold Temperatures The constant movement builds up. yes, cold water can evaporate. when liquid water meets dry air, it is not in equilibrium; water vapor is invisible. you raise an interesting point when you ask why evaporation happens more at lower temperature. Water is made up of tiny molecules that are always moving around. temperature of the. Does Water Evaporate Faster In Cold Temperatures.
From www.youtube.com
Does Hot Water Freeze Faster Than Cold Water? YouTube Does Water Evaporate Faster In Cold Temperatures Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Hot water evaporates faster than cold water because the molecules of hot water have more. temperature of the water. water vapor is invisible. for a similar reason, the air near the surface of the water will become. Does Water Evaporate Faster In Cold Temperatures.
From www.animalia-life.club
Evaporates Does Water Evaporate Faster In Cold Temperatures when liquid water meets dry air, it is not in equilibrium; a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. yes, cold water can evaporate. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure. Does Water Evaporate Faster In Cold Temperatures.
From cetovitb.blob.core.windows.net
Evaporation Temperature Definition at Marshall Taber blog Does Water Evaporate Faster In Cold Temperatures water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. The smoky white clouds rising over warm water. Does Water Evaporate Faster In Cold Temperatures.
From exokpbavp.blob.core.windows.net
Does Hot Water Boil Faster Or Cold Water at Elsie Stine blog Does Water Evaporate Faster In Cold Temperatures water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. The constant movement builds up. temperature of the water. when liquid water meets dry air, it is not in equilibrium; The smoky white clouds rising over warm water on a. Does Water Evaporate Faster In Cold Temperatures.
From 9to5science.com
[Solved] Does the temperature at which water evaporates 9to5Science Does Water Evaporate Faster In Cold Temperatures Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. temperature of the water. you raise an interesting point when you ask why evaporation happens more at lower temperature. The constant movement builds up. for a similar reason, the air near the surface of the water will. Does Water Evaporate Faster In Cold Temperatures.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition Does Water Evaporate Faster In Cold Temperatures The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. water vapor is invisible. The constant movement builds up. yes, cold water can evaporate. a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. when. Does Water Evaporate Faster In Cold Temperatures.
From exovrawwf.blob.core.windows.net
Evaporative Cooling Definition Earth Science at Charles Franklin blog Does Water Evaporate Faster In Cold Temperatures water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. you raise an interesting point when you ask why evaporation happens more at lower temperature. for a similar reason, the air near the surface of the water will become more. Does Water Evaporate Faster In Cold Temperatures.
From wxresearch.org
Does Pool Water Evaporate In Cold Weather? (The Truth!) Does Water Evaporate Faster In Cold Temperatures water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. a more mundane explanation is that hot water evaporates faster than. Does Water Evaporate Faster In Cold Temperatures.
From tipseri.com
How long does it take for water to evaporate at room temperature? Tipseri Does Water Evaporate Faster In Cold Temperatures water vapor is invisible. temperature of the water. Water is made up of tiny molecules that are always moving around. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. for a similar reason, the air near the surface of the water will become more saturated with water as. Does Water Evaporate Faster In Cold Temperatures.
From www.youtube.com
Why hot water freezes faster than cold water? With experiment Does Water Evaporate Faster In Cold Temperatures water vapor is invisible. water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. when liquid water. Does Water Evaporate Faster In Cold Temperatures.
From watermarkexplorer.blogspot.com
Evaporation Water Evaporation Temperature Does Water Evaporate Faster In Cold Temperatures for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. you raise an interesting point when you ask why evaporation happens more at lower temperature. Water is made. Does Water Evaporate Faster In Cold Temperatures.
From www.slideserve.com
PPT Water States of Matter PowerPoint Presentation, free download Does Water Evaporate Faster In Cold Temperatures temperature of the water. The constant movement builds up. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. when liquid water meets dry air, it is not in equilibrium; for a similar reason, the air near the surface of the water will become more saturated with. Does Water Evaporate Faster In Cold Temperatures.
From wxresearch.org
Does Pool Water Evaporate In Cold Weather? (The Truth!) Does Water Evaporate Faster In Cold Temperatures Hot water evaporates faster than cold water because the molecules of hot water have more. for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. water vapor is invisible. yes, cold water can evaporate. The constant movement builds up. you raise an interesting point. Does Water Evaporate Faster In Cold Temperatures.
From www.slideshare.net
Why and how hot water freezes faster than cold water Does Water Evaporate Faster In Cold Temperatures Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. yes, cold water can evaporate. for a similar reason, the air near the surface. Does Water Evaporate Faster In Cold Temperatures.
From dxoglsywy.blob.core.windows.net
How Fast Does Water Evaporate Out Of A Pool at Alfred Bailey blog Does Water Evaporate Faster In Cold Temperatures temperature of the water. water vapor is invisible. The constant movement builds up. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. Water is made up of tiny molecules that are always moving around. a more mundane explanation is that hot water evaporates faster than cold, decreasing its. Does Water Evaporate Faster In Cold Temperatures.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Water Evaporate Faster In Cold Temperatures you raise an interesting point when you ask why evaporation happens more at lower temperature. Water is made up of tiny molecules that are always moving around. when liquid water meets dry air, it is not in equilibrium; yes, cold water can evaporate. for a similar reason, the air near the surface of the water will. Does Water Evaporate Faster In Cold Temperatures.
From www.learnz.org.nz
The Water Cycle LEARNZ Does Water Evaporate Faster In Cold Temperatures you raise an interesting point when you ask why evaporation happens more at lower temperature. temperature of the water. Water is made up of tiny molecules that are always moving around. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. yes, cold water can evaporate. for a. Does Water Evaporate Faster In Cold Temperatures.
From dxouohord.blob.core.windows.net
Function Of Evaporation Water Cycle at Jonathan Martinez blog Does Water Evaporate Faster In Cold Temperatures for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. you raise an interesting point when you ask why evaporation happens more at lower temperature. The constant movement builds up. Water molecules evaporate off the surface until the amount of water in the air creates enough. Does Water Evaporate Faster In Cold Temperatures.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Water Evaporate Faster In Cold Temperatures water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. The constant movement builds up. when liquid water meets dry air, it is. Does Water Evaporate Faster In Cold Temperatures.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Does Water Evaporate Faster In Cold Temperatures Hot water evaporates faster than cold water because the molecules of hot water have more. water vapor is invisible. temperature of the water. Water is made up of tiny molecules that are always moving around. yes, cold water can evaporate. when liquid water meets dry air, it is not in equilibrium; for a similar reason,. Does Water Evaporate Faster In Cold Temperatures.
From exofcwmcx.blob.core.windows.net
Does Water Temperature Matter When Washing Dishes at Rodney Forand blog Does Water Evaporate Faster In Cold Temperatures you raise an interesting point when you ask why evaporation happens more at lower temperature. Hot water evaporates faster than cold water because the molecules of hot water have more. Water is made up of tiny molecules that are always moving around. The smoky white clouds rising over warm water on a cold day are not water vapor but. Does Water Evaporate Faster In Cold Temperatures.
From www.metoffice.gov.uk
Water cycle Met Office Does Water Evaporate Faster In Cold Temperatures you raise an interesting point when you ask why evaporation happens more at lower temperature. The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. Water is made up of tiny molecules that are always moving around. temperature of the water. The constant movement builds up. Hot water evaporates faster. Does Water Evaporate Faster In Cold Temperatures.
From www.ency123.com
Water Evaporation and Condensation Ency123 Does Water Evaporate Faster In Cold Temperatures The smoky white clouds rising over warm water on a cold day are not water vapor but liquid. water vapor is invisible. yes, cold water can evaporate. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. a more mundane explanation is that hot water evaporates faster. Does Water Evaporate Faster In Cold Temperatures.
From 9to5science.com
[Solved] Why does water evaporate at room temperature? 9to5Science Does Water Evaporate Faster In Cold Temperatures a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. when liquid water meets dry air, it is not in equilibrium; water vapor is invisible. Water is made up of tiny molecules that are always moving around. The constant movement builds up. yes,. Does Water Evaporate Faster In Cold Temperatures.
From www.slideshare.net
Why and how hot water freezes faster than cold water Does Water Evaporate Faster In Cold Temperatures yes, cold water can evaporate. for a similar reason, the air near the surface of the water will become more saturated with water as the water evaporates. a more mundane explanation is that hot water evaporates faster than cold, decreasing its volume and thus the time it takes to freeze. Hot water evaporates faster than cold water. Does Water Evaporate Faster In Cold Temperatures.