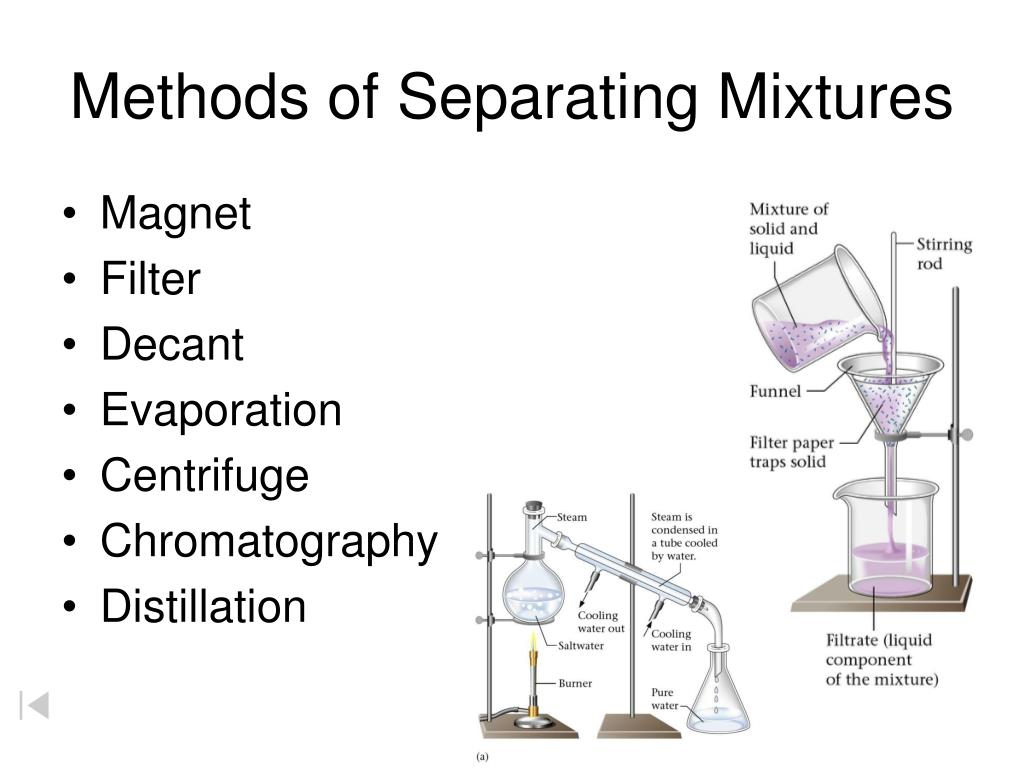
Filtration Distillation Chromatography . there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation (as discussed in analysis:. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and.
from www.slideserve.com
If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation (as discussed in analysis:. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the.
PPT Methods of Separating Mixtures PowerPoint Presentation, free
Filtration Distillation Chromatography Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation (as discussed in analysis:. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography.
From www.slideserve.com
PPT Separation Techniques PowerPoint Presentation, free download ID Filtration Distillation Chromatography there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. If two substances have different boiling points and are mixed together, you can boil them. Filtration Distillation Chromatography.
From www.youtube.com
Chromatography Types, Retention Factor, Filtration, Distillation Filtration Distillation Chromatography Distillation (as discussed in analysis:. there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. there are different ways to separate mixtures, such. Filtration Distillation Chromatography.
From aimhigh.space
Distillation and Chromatography AimHigh! Filtration Distillation Chromatography If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography.. Filtration Distillation Chromatography.
From www.youtube.com
Distillation and Gas Chromatography YouTube Filtration Distillation Chromatography Distillation (as discussed in analysis:. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. there are. Filtration Distillation Chromatography.
From www.slideserve.com
PPT CHEMISTRY I PowerPoint Presentation, free download ID3104697 Filtration Distillation Chromatography Distillation (as discussed in analysis:. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and.. Filtration Distillation Chromatography.
From eduinput.com
Can Homogeneous Mixtures be separated by Filtration? Filtration Distillation Chromatography If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. Distillation is an effective method to separate mixtures comprised of two. Filtration Distillation Chromatography.
From 12014dorcascharityng.weebly.com
FILTRATION,EVAPORATION,DISTILLATION AND PAPER CHROMATOGRAPHY 3E1 Filtration Distillation Chromatography there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. 1) the mixture is pored through a funnel lined with. Filtration Distillation Chromatography.
From thenoveldifference.com
Difference Between Distillation and Chromatography Filtration Distillation Chromatography chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. Distillation (as discussed in analysis:. there are different ways to. Filtration Distillation Chromatography.
From www.chemicals.co.uk
GCSE Chemistry Water Purification Guide The Chemistry Blog Filtration Distillation Chromatography If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. 1) the mixture is pored through a funnel lined with a filter paper, 2) the. Filtration Distillation Chromatography.
From studylib.net
Chromatography and distillation Filtration Distillation Chromatography there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips. Filtration Distillation Chromatography.
From dokumen.tips
(PPT) Filtration, Evaporation, Chromatography, Distillation DOKUMEN.TIPS Filtration Distillation Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. Distillation (as discussed in. Filtration Distillation Chromatography.
From slideplayer.com
All About Matter SC2. Obtain, evaluate, and communicate information Filtration Distillation Chromatography there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation (as discussed in analysis:. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a purification process where one. Filtration Distillation Chromatography.
From www.pinterest.co.uk
Separation techniques filtration, distillation, chromatography Filtration Distillation Chromatography Distillation (as discussed in analysis:. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. If two substances. Filtration Distillation Chromatography.
From mavink.com
Chromatography Diagram Labeled Filtration Distillation Chromatography chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. there are different ways to separate mixtures,. Filtration Distillation Chromatography.
From www.numerade.com
SOLVED QUESTION 13 The separation technique shown in the diagram is Filtration Distillation Chromatography chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation (as discussed in analysis:. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation. Filtration Distillation Chromatography.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Filtration Distillation Chromatography Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation (as discussed in analysis:. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. there are different ways to separate. Filtration Distillation Chromatography.
From www.slideserve.com
PPT Distillation & Chromatography PowerPoint Presentation, free Filtration Distillation Chromatography there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. chromatography is the separation of a mixture by passing it in solution or suspension,. Filtration Distillation Chromatography.
From www.youtube.com
Gel filtration chromatography in 5 minutes Size Exclusion Filtration Distillation Chromatography Distillation is an effective method to separate mixtures comprised of two or more pure liquids. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. there are different. Filtration Distillation Chromatography.
From www.slideserve.com
PPT Chapter 1 PowerPoint Presentation, free download ID6426283 Filtration Distillation Chromatography Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation (as discussed in analysis:. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. there are different ways to separate. Filtration Distillation Chromatography.
From www.slideserve.com
PPT Unit 1. Matter and Change PowerPoint Presentation, free download Filtration Distillation Chromatography Distillation (as discussed in analysis:. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and.. Filtration Distillation Chromatography.
From slidetodoc.com
Basic Separation Techniques Filtration Crystallization Sedimentation Filtration Distillation Chromatography If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional. Filtration Distillation Chromatography.
From www.youtube.com
Gel filtration chromatography YouTube Filtration Distillation Chromatography If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. there are different ways to separate mixtures, such as filtration,. Filtration Distillation Chromatography.
From www.slideserve.com
PPT Chapter 1 PowerPoint Presentation, free download ID6426283 Filtration Distillation Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation (as discussed in analysis:. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point. Filtration Distillation Chromatography.
From bitesizebio.com
Column Chromatography Made Simple An Easy to Follow Guide Filtration Distillation Chromatography If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. Distillation is a purification process. Filtration Distillation Chromatography.
From www.youtube.com
GCSE Chemistry 19 Which Separation Technique? Chromatography Filtration Distillation Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter. Filtration Distillation Chromatography.
From www.fishersci.com
Edvotek Principles of Gel Filtration Chromatography For 10 separations Filtration Distillation Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. Distillation (as discussed in analysis:. 1) the mixture is pored. Filtration Distillation Chromatography.
From www.xing.com
Sterile Filtration in Pharma and Biotech Manufacturing Filtration Distillation Chromatography there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a. Filtration Distillation Chromatography.
From www.youtube.com
How to separate Mixtures filtration, distillation, sublimation and Filtration Distillation Chromatography Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate. Filtration Distillation Chromatography.
From www.slideserve.com
PPT Distillation & Chromatography PowerPoint Presentation, free Filtration Distillation Chromatography Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation (as discussed in analysis:. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. 1) the mixture is pored through a funnel lined with. Filtration Distillation Chromatography.
From www.slideserve.com
PPT Methods of Separating Mixtures PowerPoint Presentation, free Filtration Distillation Chromatography there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. 1) the mixture. Filtration Distillation Chromatography.
From video.yckmc.edu.hk
Chemistry tutorialCh621Filtration, crystallization, simple Filtration Distillation Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. If two substances have different boiling points and are mixed together, you can boil them and the one with the lower boiling point will evaporate out. Distillation is. Filtration Distillation Chromatography.
From mavink.com
Chromatography Diagram Labeled Filtration Distillation Chromatography 1) the mixture is pored through a funnel lined with a filter paper, 2) the filtrate (liquid) drips through to the filter flask, 3) the solid remains in the. there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation. Filtration Distillation Chromatography.
From www.studocu.com
Separative Methods in Chemistry Filtration Distillation Chromatography Filtration Distillation Chromatography Distillation (as discussed in analysis:. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. chromatography is the separation of a mixture by passing it in solution or suspension, or as a vapor (as in gas chromatography),. If. Filtration Distillation Chromatography.
From www.vrogue.co
Types Of Chromatography Used In Bioprocessing Design vrogue.co Filtration Distillation Chromatography there are different ways to separate mixtures, eg by filtration, crystallisation, distillation or chromatography. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation (as discussed in analysis:. chromatography is the separation of a mixture. Filtration Distillation Chromatography.
From www.slideserve.com
PPT Classification of PowerPoint Presentation, free download ID9528819 Filtration Distillation Chromatography there are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. Distillation (as discussed in analysis:. there are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. there are. Filtration Distillation Chromatography.