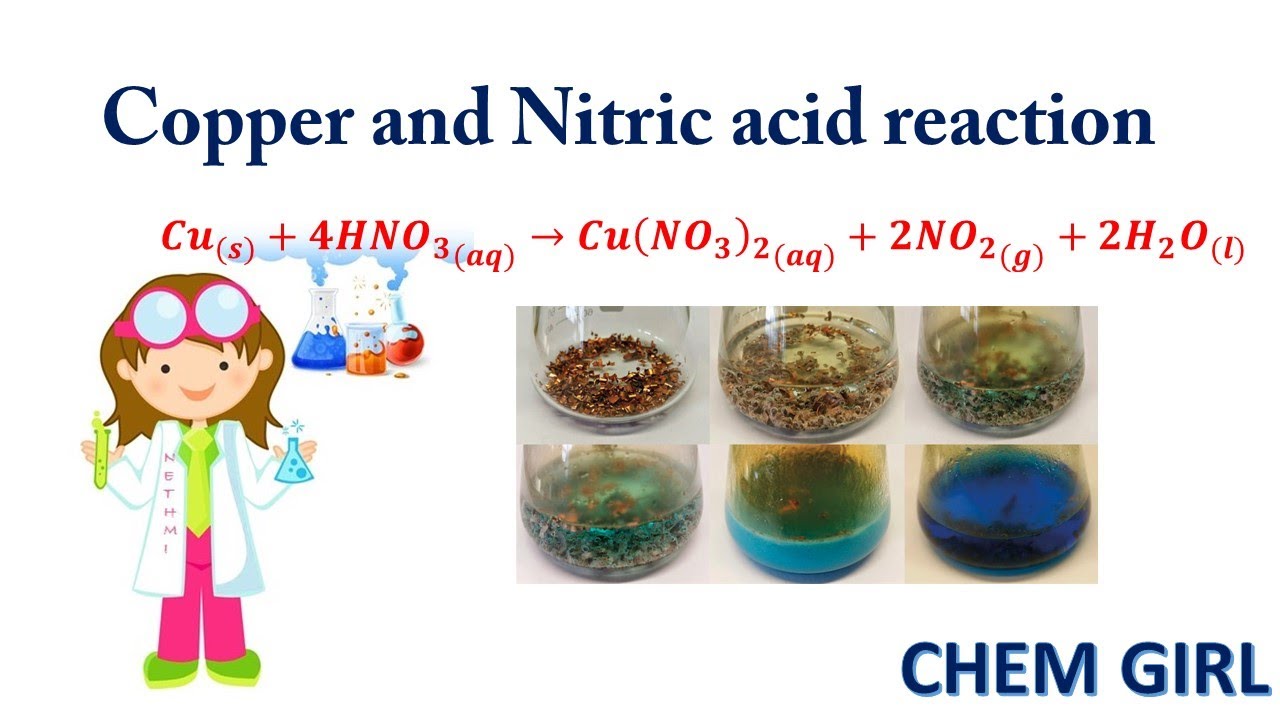
Copper Chloride + Nitric Acid . Declan fleming shows how copper can in fact react with some acids by demonstrating the. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. a more complex redox reaction occurs when copper dissolves in nitric acid. dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. the reaction proceeds as: 655k views 12 years ago education. royal society of chemistry. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide.
from www.youtube.com
a more complex redox reaction occurs when copper dissolves in nitric acid. dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. Declan fleming shows how copper can in fact react with some acids by demonstrating the. 655k views 12 years ago education. royal society of chemistry. the reaction proceeds as:
Copper and Nitric acid reaction YouTube
Copper Chloride + Nitric Acid 655k views 12 years ago education. Declan fleming shows how copper can in fact react with some acids by demonstrating the. the reaction proceeds as: In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. royal society of chemistry. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. a more complex redox reaction occurs when copper dissolves in nitric acid. 655k views 12 years ago education.
From sciencenotes.org
Copper and Nitric Acid Chemistry Demonstration Copper Chloride + Nitric Acid if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Declan fleming shows how copper can in fact react with some acids by demonstrating the. dilute nitric. Copper Chloride + Nitric Acid.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper Copper Chloride + Nitric Acid a more complex redox reaction occurs when copper dissolves in nitric acid. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. 655k views 12 years ago education. royal society of chemistry. the reaction proceeds as: Declan fleming shows how copper. Copper Chloride + Nitric Acid.
From studylib.net
Laboratory 5 Stoichiometry of Copper (II) Chloride and Aluminum Copper Chloride + Nitric Acid the reaction proceeds as: Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. a more complex redox reaction occurs when copper dissolves in nitric acid. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown. Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Chloride + Nitric Acid In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h. Copper Chloride + Nitric Acid.
From www.sciencephoto.com
Copper reacting with nitric acid Stock Image C023/0596 Science Copper Chloride + Nitric Acid a more complex redox reaction occurs when copper dissolves in nitric acid. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. royal society of chemistry. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide.. Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Chloride + Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. a more complex redox reaction occurs when copper dissolves in nitric acid. the reaction proceeds as: Declan fleming shows how copper can in fact react with some acids by demonstrating the. . Copper Chloride + Nitric Acid.
From www.flickr.com
copper/nitric acid reaction chemicalarts Flickr Copper Chloride + Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. a more complex redox reaction occurs when copper dissolves in nitric acid. 655k views 12 years ago education. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the. Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
copper nitric acid reaction redox oxidation reduction Fundamental Copper Chloride + Nitric Acid royal society of chemistry. a more complex redox reaction occurs when copper dissolves in nitric acid. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. the reaction proceeds as: 655k views 12 years ago education. Declan fleming shows. Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
copper nitric acid reaction redox oxidation reduction Fundamental Copper Chloride + Nitric Acid royal society of chemistry. 655k views 12 years ago education. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. the reaction proceeds as: Declan fleming. Copper Chloride + Nitric Acid.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Copper Chloride + Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. 655k views 12 years ago education. a more complex redox reaction occurs when copper dissolves in nitric acid. . Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Chloride + Nitric Acid if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. royal society of chemistry. the reaction proceeds as: In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. a more complex redox reaction occurs when. Copper Chloride + Nitric Acid.
From www.numerade.com
⏩SOLVEDWhen solid copper is added to nitric acid, copper(II)… Numerade Copper Chloride + Nitric Acid a more complex redox reaction occurs when copper dissolves in nitric acid. the reaction proceeds as: royal society of chemistry. 655k views 12 years ago education. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base. Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Chloride + Nitric Acid a more complex redox reaction occurs when copper dissolves in nitric acid. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. the reaction proceeds as: Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no. Copper Chloride + Nitric Acid.
From www.sciencephoto.com
Copper Reacts With Nitric Acid Stock Image C001/7513 Science Copper Chloride + Nitric Acid dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow. Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Chloride + Nitric Acid Declan fleming shows how copper can in fact react with some acids by demonstrating the. royal society of chemistry. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. a more complex redox reaction occurs when copper dissolves in nitric acid. . Copper Chloride + Nitric Acid.
From www.youtube.com
Reaction of Nitric Acid with Metals, Chemistry Lecture Sabaq.pk YouTube Copper Chloride + Nitric Acid dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Declan fleming shows how copper can in fact react with some acids by demonstrating the. a more complex redox reaction occurs when copper dissolves in nitric acid. if solutions of fe(iii) salts are acidified. Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Chloride + Nitric Acid dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. 655k views 12 years ago education. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. royal society of. Copper Chloride + Nitric Acid.
From www.alamy.com
Concentrated Nitric Acid Reacting with Copper and Generating Nitrogen Copper Chloride + Nitric Acid the reaction proceeds as: In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. royal society of chemistry. if solutions of fe(iii) salts are acidified with perchloric acid or. Copper Chloride + Nitric Acid.
From www.sciencephoto.com
Copper reacts with nitric acid, 1 of 3 Stock Image C044/5795 Copper Chloride + Nitric Acid Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. the reaction proceeds as: Declan fleming shows how copper can in fact react with some acids by demonstrating the.. Copper Chloride + Nitric Acid.
From sea-hill.co.th
100456 Nitric acid 65 seahill.co.th Copper Chloride + Nitric Acid In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. a more complex redox reaction occurs when copper dissolves in nitric acid. 655k views 12 years ago education. royal society of chemistry. the reaction proceeds as: dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ),. Copper Chloride + Nitric Acid.
From www.youtube.com
Copper Penny reacting with Nitric Acid YouTube Copper Chloride + Nitric Acid a more complex redox reaction occurs when copper dissolves in nitric acid. dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Declan fleming shows how copper can in fact react with some acids by demonstrating the. the reaction proceeds as: Cu (s) +. Copper Chloride + Nitric Acid.
From sciencenotes.org
Copper and Nitric Acid Chemistry Demonstration Copper Chloride + Nitric Acid the reaction proceeds as: Declan fleming shows how copper can in fact react with some acids by demonstrating the. a more complex redox reaction occurs when copper dissolves in nitric acid. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution. Copper Chloride + Nitric Acid.
From www.sciencephoto.com
Copper reacts with nitric acid Stock Image C044/5778 Science Copper Chloride + Nitric Acid 655k views 12 years ago education. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. Declan fleming shows how copper can in fact react with some acids by demonstrating. Copper Chloride + Nitric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Copper Chloride + Nitric Acid Declan fleming shows how copper can in fact react with some acids by demonstrating the. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated. Copper Chloride + Nitric Acid.
From fphoto.photoshelter.com
science chemistry oxidation reaction copper nitric acid Fundamental Copper Chloride + Nitric Acid dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Declan fleming shows how copper can in fact react with some acids by demonstrating the. 655k views 12 years ago education. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2. Copper Chloride + Nitric Acid.
From www.youtube.com
90 g of a silver coin was dissolved in strong nitric acid and excess of Copper Chloride + Nitric Acid royal society of chemistry. Declan fleming shows how copper can in fact react with some acids by demonstrating the. dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown. Copper Chloride + Nitric Acid.
From edu.rsc.org
Dissolving copper in nitric acid Exhibition chemistry RSC Education Copper Chloride + Nitric Acid 655k views 12 years ago education. a more complex redox reaction occurs when copper dissolves in nitric acid. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. Declan fleming shows how copper can in fact react with some acids by demonstrating the.. Copper Chloride + Nitric Acid.
From www.youtube.com
Nitric acid, Copper and Zinc YouTube Copper Chloride + Nitric Acid Declan fleming shows how copper can in fact react with some acids by demonstrating the. In this reaction, copper is oxidized while nitric acid is reduced to nitric oxide. the reaction proceeds as: if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the. Copper Chloride + Nitric Acid.
From www.alamy.com
Copper and nitric acid reacting in beaker to form nitrogen dioxide gas Copper Chloride + Nitric Acid dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. a more complex redox reaction occurs when copper dissolves. Copper Chloride + Nitric Acid.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Chloride + Nitric Acid dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. In this reaction, copper is oxidized while nitric acid is. Copper Chloride + Nitric Acid.
From www.sciencephoto.com
Reaction of Copper and Nitric Acid Stock Image C004/7007 Science Copper Chloride + Nitric Acid Declan fleming shows how copper can in fact react with some acids by demonstrating the. a more complex redox reaction occurs when copper dissolves in nitric acid. Cu (s) + 4hno 3 (aq) → cu (no 3) 2 (aq) + 2no 2 (g) + 2h 2 o (l) the initial green colour is. if solutions of fe(iii) salts. Copper Chloride + Nitric Acid.
From www.numerade.com
SOLVED How many grams of copper (I) chloride are formed if 5.000 g of Copper Chloride + Nitric Acid the reaction proceeds as: royal society of chemistry. dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. a more complex redox reaction occurs when copper dissolves in nitric acid. if solutions of fe(iii) salts are acidified with perchloric acid or nitric. Copper Chloride + Nitric Acid.
From cymitquimica.com
Reagecon Copper Standard for Atomic Absorption (AAS) 100 µg/mL (100 ppm Copper Chloride + Nitric Acid if solutions of fe(iii) salts are acidified with perchloric acid or nitric acid, the brown base is protonated and the yellow color disappears from the solution entirely. Declan fleming shows how copper can in fact react with some acids by demonstrating the. a more complex redox reaction occurs when copper dissolves in nitric acid. Cu (s) + 4hno. Copper Chloride + Nitric Acid.
From www.youtube.com
Copper and Nitric acid reaction YouTube Copper Chloride + Nitric Acid dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. Declan fleming shows how copper can in fact react with some acids by demonstrating the. the reaction proceeds as: 655k views 12 years ago education. Cu (s) + 4hno 3 (aq) → cu (no 3). Copper Chloride + Nitric Acid.
From www.sciencephoto.com
Copper Reacts with Nitric Acid Stock Image C028/1266 Science Copper Chloride + Nitric Acid a more complex redox reaction occurs when copper dissolves in nitric acid. Declan fleming shows how copper can in fact react with some acids by demonstrating the. dilute nitric acid reacts with copper and produce copper nitrate ( cu (no 3) 2 ), nitric oxide (no) and water as products. the reaction proceeds as: 655k views 12. Copper Chloride + Nitric Acid.