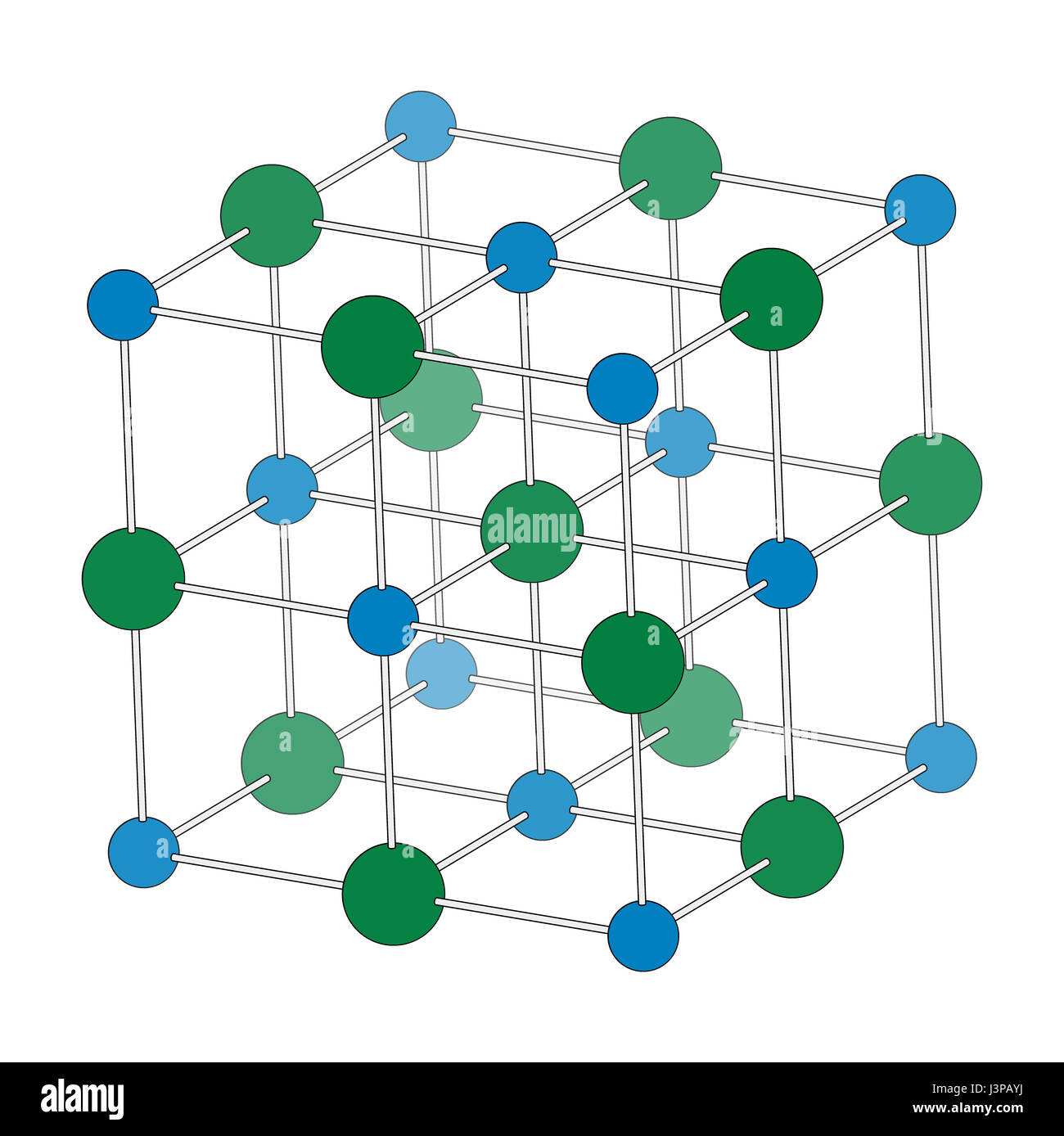
Table Salt Chloride Atoms . sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. the molecular weight of nacl is 58.44g/mol. Compounds with the sodium chloride structure include alikali. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. These ions are na +. table salt is an ionic compound, which breaks into its component ions or dissociates in water.
from www.alamy.com
sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. the molecular weight of nacl is 58.44g/mol. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. table salt is an ionic compound, which breaks into its component ions or dissociates in water. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. These ions are na +. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. Compounds with the sodium chloride structure include alikali.
Sodium chloride lattice Cut Out Stock Images & Pictures Alamy
Table Salt Chloride Atoms These ions are na +. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. the molecular weight of nacl is 58.44g/mol. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. table salt is an ionic compound, which breaks into its component ions or dissociates in water. These ions are na +. Compounds with the sodium chloride structure include alikali.
From mungfali.com
Sodium Chloride Molecule Diagram Table Salt Chloride Atoms Compounds with the sodium chloride structure include alikali. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. These ions are na +. table. Table Salt Chloride Atoms.
From cartoondealer.com
Sodium Chloride Rock Salt, Halite, Table Salt, Chemical Structure Table Salt Chloride Atoms sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. the molecular weight of nacl is 58.44g/mol. it has 1:1 stoichiometry ratio of na:cl with a molar. Table Salt Chloride Atoms.
From www.alamy.com
Sodium chloride (NaCl, table salt), crystal structure. Atoms are Table Salt Chloride Atoms an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. These ions are na +. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. Compounds with the sodium chloride structure include alikali. the molecular weight of nacl is 58.44g/mol.. Table Salt Chloride Atoms.
From www.alamy.com
Sodium chloride (rock salt, halite, table salt), crystal structure Table Salt Chloride Atoms an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol.. Table Salt Chloride Atoms.
From www.thoughtco.com
Table Salt Molecular Formula Sodium Chloride Table Salt Chloride Atoms an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. table salt is an ionic compound, which breaks into its component ions or dissociates in water. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very. Table Salt Chloride Atoms.
From www.dreamstime.com
Sodium Chloride (rock Salt, Halite, Table Salt), Crystal Structure Table Salt Chloride Atoms Compounds with the sodium chloride structure include alikali. These ions are na +. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. it. Table Salt Chloride Atoms.
From www.alamy.com
Sodium chloride (rock salt, halite, table salt), crystal structure Table Salt Chloride Atoms Compounds with the sodium chloride structure include alikali. table salt is an ionic compound, which breaks into its component ions or dissociates in water. These ions are na +. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. the molecular weight of nacl is 58.44g/mol. . Table Salt Chloride Atoms.
From www.youtube.com
How to Find the Number of Atoms in NaCl (Sodium chloride) YouTube Table Salt Chloride Atoms it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. an atom of. Table Salt Chloride Atoms.
From www.alamy.com
Sodium chloride (rock salt, halite, table salt), crystal structure Table Salt Chloride Atoms an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and.. Table Salt Chloride Atoms.
From www.animalia-life.club
Table Salt Chemical Structure Table Salt Chloride Atoms sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. Compounds with the sodium chloride structure include alikali. the molecular weight of nacl is 58.44g/mol. table salt. Table Salt Chloride Atoms.
From www.alamy.com
Chlorine Atomic Structure Stock Photos & Chlorine Atomic Structure Table Salt Chloride Atoms sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. These ions are na +. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. it has 1:1 stoichiometry ratio of na:cl with a molar. Table Salt Chloride Atoms.
From www.dreamstime.com
Sodium Chloride (rock Salt, Halite, Table Salt), Crystal Structure Table Salt Chloride Atoms Compounds with the sodium chloride structure include alikali. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. These ions are na +. table salt is an ionic compound, which breaks into its component ions or dissociates in water. sodium chloride, nacl the classic. Table Salt Chloride Atoms.
From cartoondealer.com
Sodium Chloride (rock Salt, Halite, Table Salt), Crystal Structure Table Salt Chloride Atoms sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. the molecular weight of nacl is 58.44g/mol. table salt is an ionic compound, which breaks into its component ions or dissociates in water. an atom of sodium (na) donates one of its electrons to an atom. Table Salt Chloride Atoms.
From cabinet.matttroy.net
Is Table Salt A Molecule Or Compound Matttroy Table Salt Chloride Atoms These ions are na +. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. Compounds with the sodium chloride structure include alikali. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. sodium. Table Salt Chloride Atoms.
From www.alamy.com
Crystal structure of Sodium chloride and diatomic molecule of salt Table Salt Chloride Atoms table salt is an ionic compound, which breaks into its component ions or dissociates in water. Compounds with the sodium chloride structure include alikali. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical. Table Salt Chloride Atoms.
From www.dreamstime.com
Sodium Chloride (rock Salt, Halite, Table Salt), Crystal Structure Table Salt Chloride Atoms sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. These ions are na +. Compounds with the sodium chloride structure include alikali. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. table salt is an ionic. Table Salt Chloride Atoms.
From www.alamy.com
Sodium chloride (NaCl, table salt), crystal structure. Atoms are Table Salt Chloride Atoms These ions are na +. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. the molecular weight of nacl is 58.44g/mol. Compounds with the sodium chloride structure include alikali. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms. Table Salt Chloride Atoms.
From www.alamy.com
Nacl crystal lattice hires stock photography and images Alamy Table Salt Chloride Atoms table salt is an ionic compound, which breaks into its component ions or dissociates in water. These ions are na +. Compounds with the sodium chloride structure include alikali. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. the molecular weight of nacl is 58.44g/mol. . Table Salt Chloride Atoms.
From montessorimuddle.org
subatomic particles Montessori Muddle Table Salt Chloride Atoms the molecular weight of nacl is 58.44g/mol. table salt is an ionic compound, which breaks into its component ions or dissociates in water. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. Compounds with the sodium chloride structure include alikali. sodium atoms (and all metals in general) have a weak hold. Table Salt Chloride Atoms.
From www.alamy.com
Sodium chloride structure hires stock photography and images Alamy Table Salt Chloride Atoms the molecular weight of nacl is 58.44g/mol. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. These ions are na +. sodium chloride, nacl the classic case of. Table Salt Chloride Atoms.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Table Salt Chloride Atoms table salt is an ionic compound, which breaks into its component ions or dissociates in water. These ions are na +. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. an atom. Table Salt Chloride Atoms.
From www.universetoday.com
Earth is the Most Exotic Place In The Universe Table Salt Chloride Atoms These ions are na +. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. Compounds with the sodium chloride structure include alikali. sodium chloride, nacl the classic case of ionic bonding, the sodium. Table Salt Chloride Atoms.
From www.alamy.com
Sodium chloride structure hires stock photography and images Alamy Table Salt Chloride Atoms These ions are na +. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. table salt is an ionic compound, which breaks into its component ions or dissociates in water. the molecular weight of nacl is 58.44g/mol. it has 1:1 stoichiometry ratio. Table Salt Chloride Atoms.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Table Salt Chloride Atoms These ions are na +. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. table salt is an ionic compound, which breaks into its component ions or. Table Salt Chloride Atoms.
From mydiagram.online
[DIAGRAM] Water And Sodium Chloride Molecular Diagram Table Salt Chloride Atoms Compounds with the sodium chloride structure include alikali. table salt is an ionic compound, which breaks into its component ions or dissociates in water. the molecular weight of nacl is 58.44g/mol. sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. an atom of sodium (na). Table Salt Chloride Atoms.
From www.animalia-life.club
Table Salt Chemical Structure Table Salt Chloride Atoms an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. table salt is an ionic compound, which breaks into its component ions or dissociates in water. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. sodium chloride, nacl. Table Salt Chloride Atoms.
From www.dreamstime.com
Sodium Chloride (rock Salt, Halite, Table Salt), Crystal Structure Table Salt Chloride Atoms Compounds with the sodium chloride structure include alikali. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. These ions are na +. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. table salt is an ionic compound, which breaks into its component. Table Salt Chloride Atoms.
From www.vecteezy.com
Chemistry model salt molecule diatomic sodium chlorine NaCl scientific Table Salt Chloride Atoms sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. These ions are na +. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very. Table Salt Chloride Atoms.
From www.shutterstock.com
Sodium Chloride (Rock Salt, Halite, Table Salt), Crystal Structure Table Salt Chloride Atoms table salt is an ionic compound, which breaks into its component ions or dissociates in water. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. These ions are na +. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in. Table Salt Chloride Atoms.
From awesomehome.co
Is Table Salt A Compound Awesome Home Table Salt Chloride Atoms sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. the molecular weight of nacl is 58.44g/mol. table salt is an ionic compound, which breaks into its component ions or dissociates in water.. Table Salt Chloride Atoms.
From www.turfcaresupply.com
JOE KNOWS! Cation Exchange Capacity Table Salt Chloride Atoms sodium chloride, nacl the classic case of ionic bonding, the sodium chloride molecule forms by the ionization of sodium and. Compounds with the sodium chloride structure include alikali. These ions are na +. the molecular weight of nacl is 58.44g/mol. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in. Table Salt Chloride Atoms.
From chemistrylearnwithsangam.blogspot.com
chemistry knowledge Comparison between Covalent and Ionic Bond Table Salt Chloride Atoms the molecular weight of nacl is 58.44g/mol. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. it has 1:1 stoichiometry ratio of na:cl. Table Salt Chloride Atoms.
From www.alamy.com
Sodium chloride lattice Cut Out Stock Images & Pictures Alamy Table Salt Chloride Atoms table salt is an ionic compound, which breaks into its component ions or dissociates in water. the molecular weight of nacl is 58.44g/mol. sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. an atom of sodium (na) donates one of its electrons to an atom of. Table Salt Chloride Atoms.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Table Salt Chloride Atoms These ions are na +. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. it has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. Compounds with the sodium chloride structure include alikali. sodium atoms (and all metals in general). Table Salt Chloride Atoms.
From sphweb.bumc.bu.edu
Chemical Elements Atoms Table Salt Chloride Atoms sodium atoms (and all metals in general) have a weak hold on electrons, whereas chlorine atoms are very electronegative. the molecular weight of nacl is 58.44g/mol. an atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. it has 1:1 stoichiometry ratio of na:cl. Table Salt Chloride Atoms.