Lead Nuclear Charge . Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 133 rows lead (82 pb) has four observationally stable isotopes: See all 15 effective nuclear charges. Electrons are attracted to the nucleus as it is. 204 pb, 206 pb, 207 pb, 208 pb. Z eff = ζ × n. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge.
from www.youtube.com
Electrons are attracted to the nucleus as it is. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. 133 rows lead (82 pb) has four observationally stable isotopes: 204 pb, 206 pb, 207 pb, 208 pb. Z eff = ζ × n. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. See all 15 effective nuclear charges.
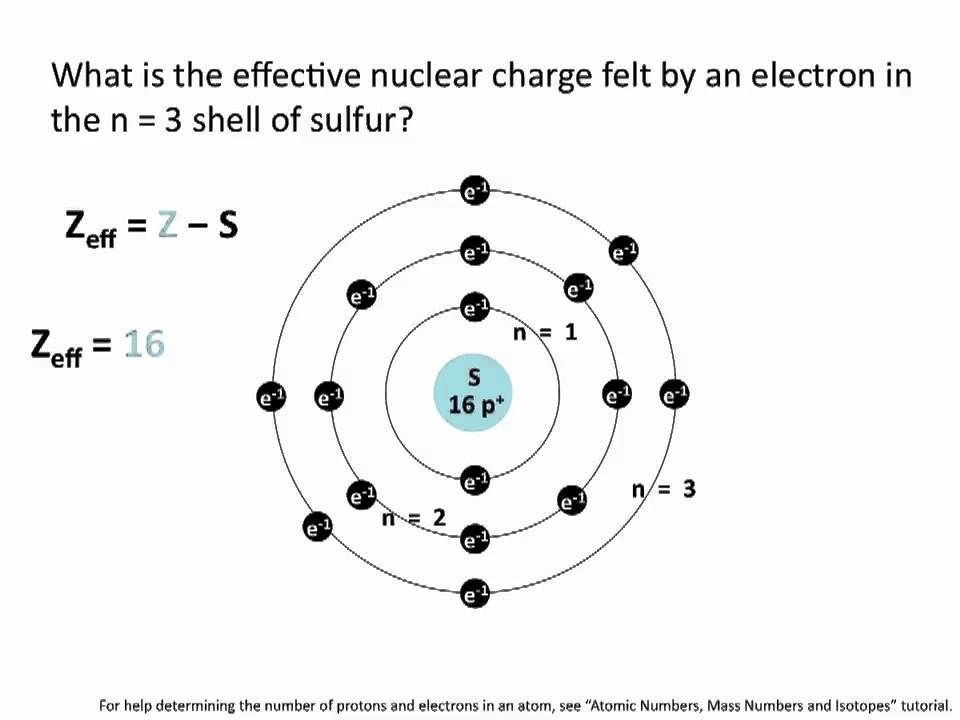
Effective Nuclear Charge Chemistry Tutorial YouTube
Lead Nuclear Charge Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 133 rows lead (82 pb) has four observationally stable isotopes: 204 pb, 206 pb, 207 pb, 208 pb. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. See all 15 effective nuclear charges. Electrons are attracted to the nucleus as it is. Z eff = ζ × n. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus.
From www.pinterest.com
What is Nuclear Charge with Examples ,Trend & important questions Lead Nuclear Charge See all 15 effective nuclear charges. Electrons are attracted to the nucleus as it is. 204 pb, 206 pb, 207 pb, 208 pb. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear. Lead Nuclear Charge.
From www.breakingatom.com
Nuclear charge Definition Lead Nuclear Charge The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. Electrons are attracted to the nucleus as it is. 133 rows lead (82 pb) has four observationally stable isotopes: See all 15. Lead Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge for Intro Chem Students.wmv YouTube Lead Nuclear Charge See all 15 effective nuclear charges. Z eff = ζ × n. 133 rows lead (82 pb) has four observationally stable isotopes: Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell,. Lead Nuclear Charge.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Lead Nuclear Charge Z eff = ζ × n. 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. Electrons are attracted to the nucleus. Lead Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Lead Nuclear Charge 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. See all 15 effective nuclear charges. Electrons are attracted to the nucleus. Lead Nuclear Charge.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Nuclear Charge Electrons are attracted to the nucleus as it is. Z eff = ζ × n. 133 rows lead (82 pb) has four observationally stable isotopes: Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 204 pb, 206 pb, 207 pb, 208 pb. The effective nuclear charge. Lead Nuclear Charge.
From chem.libretexts.org
1.15 Effective Nuclear Charge and Shielding Chemistry LibreTexts Lead Nuclear Charge 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. Z eff = ζ × n. Electrons are attracted to the nucleus. Lead Nuclear Charge.
From pediaa.com
Difference Between Nuclear Charge and Effective Nuclear Charge Lead Nuclear Charge Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 204 pb, 206 pb, 207 pb, 208 pb. Electrons are attracted to the nucleus as it is. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because. Lead Nuclear Charge.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE Lead Nuclear Charge 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. See all 15 effective nuclear charges. Nuclear charge is a measure of. Lead Nuclear Charge.
From www.quora.com
How does an increase in nuclear charge lead to a decrease in ionization Lead Nuclear Charge Z eff = ζ × n. 133 rows lead (82 pb) has four observationally stable isotopes: Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. Electrons are attracted to the nucleus as it is. 204 pb, 206 pb, 207 pb, 208 pb. The effective nuclear charge. Lead Nuclear Charge.
From www.youtube.com
CFD Analysis of a LeadCooled Nuclear Reactor YouTube Lead Nuclear Charge The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. 204 pb, 206 pb, 207 pb, 208 pb. See all 15 effective nuclear charges. Electrons are attracted to the nucleus as it. Lead Nuclear Charge.
From www.youtube.com
Nuclear Charge and Effective Nuclear Charge YouTube Lead Nuclear Charge 133 rows lead (82 pb) has four observationally stable isotopes: Electrons are attracted to the nucleus as it is. 204 pb, 206 pb, 207 pb, 208 pb. See all 15 effective nuclear charges. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly. Lead Nuclear Charge.
From www.env.go.jp
Power of Radiation [MOE] Lead Nuclear Charge See all 15 effective nuclear charges. 133 rows lead (82 pb) has four observationally stable isotopes: 204 pb, 206 pb, 207 pb, 208 pb. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. Electrons are attracted to the nucleus as it is. The effective nuclear charge. Lead Nuclear Charge.
From facts.net
11 Captivating Facts About Effective Nuclear Charge Lead Nuclear Charge See all 15 effective nuclear charges. 204 pb, 206 pb, 207 pb, 208 pb. Electrons are attracted to the nucleus as it is. 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly. Lead Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Periodicity and Atomic Structure PowerPoint Lead Nuclear Charge 204 pb, 206 pb, 207 pb, 208 pb. 133 rows lead (82 pb) has four observationally stable isotopes: Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. See all 15 effective nuclear charges. Electrons are attracted to the nucleus as it is. The effective nuclear charge. Lead Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps Lead Nuclear Charge The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. Electrons are attracted to the nucleus as it is. 204 pb, 206 pb, 207 pb, 208 pb. See all 15 effective nuclear. Lead Nuclear Charge.
From www.chegg.com
Solved Are the effective nuclear charges listed in Figure Lead Nuclear Charge 133 rows lead (82 pb) has four observationally stable isotopes: Z eff = ζ × n. 204 pb, 206 pb, 207 pb, 208 pb. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the. Lead Nuclear Charge.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2559808 Lead Nuclear Charge Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. Electrons are attracted to the nucleus as it is. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective. Lead Nuclear Charge.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead Nuclear Charge 204 pb, 206 pb, 207 pb, 208 pb. See all 15 effective nuclear charges. Electrons are attracted to the nucleus as it is. Z eff = ζ × n. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons. Lead Nuclear Charge.
From www.youtube.com
How To Calculate Effective Nuclear Charge YouTube Lead Nuclear Charge 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. Nuclear charge is a measure of the ability of protons in the. Lead Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube Lead Nuclear Charge Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. Z eff = ζ × n. 204 pb, 206 pb, 207 pb, 208 pb. 133 rows lead (82 pb) has four observationally stable isotopes: Electrons are attracted to the nucleus as it is. The effective nuclear charge. Lead Nuclear Charge.
From www.slideserve.com
PPT Effective Nuclear Charge PowerPoint Presentation, free download Lead Nuclear Charge 204 pb, 206 pb, 207 pb, 208 pb. See all 15 effective nuclear charges. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. Lead Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge and Periodic Trends YouTube Lead Nuclear Charge 204 pb, 206 pb, 207 pb, 208 pb. Z eff = ζ × n. See all 15 effective nuclear charges. 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding. Lead Nuclear Charge.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead Nuclear Charge Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 133 rows lead (82 pb) has four observationally stable isotopes: See all 15 effective nuclear charges. 204 pb, 206 pb, 207 pb, 208 pb. Z eff = ζ × n. Electrons are attracted to the nucleus as. Lead Nuclear Charge.
From studylib.net
Effective Nuclear Charge Lead Nuclear Charge See all 15 effective nuclear charges. 204 pb, 206 pb, 207 pb, 208 pb. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled. Lead Nuclear Charge.
From www.slideserve.com
PPT Periodic Properties of the Elements PowerPoint Presentation, free Lead Nuclear Charge Z eff = ζ × n. 204 pb, 206 pb, 207 pb, 208 pb. See all 15 effective nuclear charges. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little. Lead Nuclear Charge.
From www.slideserve.com
PPT Chapter 7. Periodic Properties of the Elements. PowerPoint Lead Nuclear Charge See all 15 effective nuclear charges. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. 204 pb, 206 pb, 207 pb, 208 pb. Z eff = ζ × n. Nuclear charge. Lead Nuclear Charge.
From www.breakingatom.com
Nuclear Charge Lead Nuclear Charge Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. See all 15 effective nuclear charges. Electrons are attracted to the nucleus as it is. 204 pb, 206 pb, 207 pb, 208 pb. The effective nuclear charge changes relatively little for electrons in the outermost, or valence. Lead Nuclear Charge.
From www.numerade.com
SOLVEDDefine shielding and effective nuclear charge. What is the Lead Nuclear Charge See all 15 effective nuclear charges. Electrons are attracted to the nucleus as it is. 133 rows lead (82 pb) has four observationally stable isotopes: Z eff = ζ × n. 204 pb, 206 pb, 207 pb, 208 pb. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around. Lead Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Lead Nuclear Charge See all 15 effective nuclear charges. Z eff = ζ × n. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 133 rows lead (82 pb) has four observationally stable isotopes: The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell,. Lead Nuclear Charge.
From www.youtube.com
How To Calculate The Effective Nuclear Charge of an Electron YouTube Lead Nuclear Charge Electrons are attracted to the nucleus as it is. 133 rows lead (82 pb) has four observationally stable isotopes: 204 pb, 206 pb, 207 pb, 208 pb. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. See all 15 effective nuclear charges. Z eff = ζ. Lead Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps Lead Nuclear Charge 204 pb, 206 pb, 207 pb, 208 pb. 133 rows lead (82 pb) has four observationally stable isotopes: Electrons are attracted to the nucleus as it is. See all 15 effective nuclear charges. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. Z eff = ζ. Lead Nuclear Charge.
From www.breakingatom.com
Nuclear Charge Lead Nuclear Charge Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 133 rows lead (82 pb) has four observationally stable isotopes: 204 pb, 206 pb, 207 pb, 208 pb. Electrons are attracted to the nucleus as it is. See all 15 effective nuclear charges. Z eff = ζ. Lead Nuclear Charge.
From www.youtube.com
Difference between Nuclear Charge and Effective Nuclear Charge Lead Nuclear Charge See all 15 effective nuclear charges. The effective nuclear charge changes relatively little for electrons in the outermost, or valence shell, from lithium to cesium because electrons in filled inner shells are highly effective at shielding electrons in outer shells from the nuclear charge. Z eff = ζ × n. Nuclear charge is a measure of the ability of protons. Lead Nuclear Charge.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Lead Nuclear Charge 133 rows lead (82 pb) has four observationally stable isotopes: Z eff = ζ × n. See all 15 effective nuclear charges. Nuclear charge is a measure of the ability of protons in the nucleus to attract the negative electrons in orbit around the nucleus. 204 pb, 206 pb, 207 pb, 208 pb. The effective nuclear charge changes relatively little. Lead Nuclear Charge.