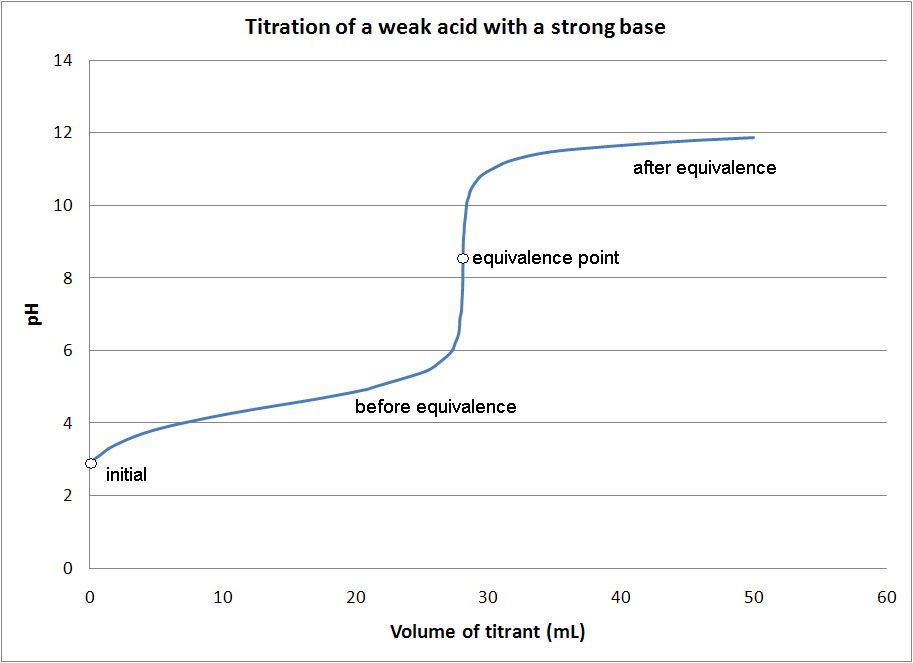
Titration Curves A Level Chemistry . It involves adding a titrant of known concentration. The end point of a titration is the point at which an indicator changes. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. What are ph titration curves. A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; A titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.
from www.writework.com
Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. It involves adding a titrant of known concentration. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. What are ph titration curves. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.
Titration of amino acids WriteWork
Titration Curves A Level Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. It involves adding a titrant of known concentration. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. What are ph titration curves. The end point of a titration is the point at which an indicator changes.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Curves A Level Chemistry The end point of a titration is the point at which an indicator changes. It involves adding a titrant of known concentration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the. Titration Curves A Level Chemistry.
From www.youtube.com
TRU Chemistry labs How To Plot a Titration Curve YouTube Titration Curves A Level Chemistry A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the. Titration Curves A Level Chemistry.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curves A Level Chemistry A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. What are ph titration curves. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A titration curve is a graphical representation of the ph of a solution during a titration.. Titration Curves A Level Chemistry.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curves A Level Chemistry The end point of a titration is the point at which an indicator changes. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Without titles for the. Titration Curves A Level Chemistry.
From mavink.com
Strong Acid And Base Titration Curve Titration Curves A Level Chemistry The end point of a titration is the point at which an indicator changes. What are ph titration curves. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph. Titration Curves A Level Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Curves A Level Chemistry A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. What are ph titration curves. Without titles for the graph you can easily recognise which combination is shown by. Titration Curves A Level Chemistry.
From www.revisely.co.uk
ALevel AQA Chemistry Questions pH Curves, Titrations and Indicators Titration Curves A Level Chemistry Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; The end point of a titration is the point at which an indicator changes. What are ph titration curves. A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. It involves. Titration Curves A Level Chemistry.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curves A Level Chemistry Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. What are ph titration curves. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. It involves adding. Titration Curves A Level Chemistry.
From www.savemyexams.com
pH Titration Curves OCR A Level Chemistry Revision Notes 2017 Titration Curves A Level Chemistry What are ph titration curves. A titration curve is a graphical representation of the ph of a solution during a titration. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Without titles for the graph you can easily recognise which combination is shown by looking at the. Titration Curves A Level Chemistry.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Curves A Level Chemistry A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. What are ph titration curves. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A ph curve is a graphical representation that plots the ph of the solution being titrated. Titration Curves A Level Chemistry.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Curves A Level Chemistry Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph. Titration Curves A Level Chemistry.
From learnah.org
5 Titration curves and Indicators Titration Curves A Level Chemistry The end point of a titration is the point at which an indicator changes. It involves adding a titrant of known concentration. A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown. Titration Curves A Level Chemistry.
From www.writework.com
Titration of amino acids WriteWork Titration Curves A Level Chemistry Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. The end point of a. Titration Curves A Level Chemistry.
From opentextbc.ca
14.7 AcidBase Titrations Chemistry Titration Curves A Level Chemistry A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve can be used to determine the pka or pkb of an unknown. Titration Curves A Level Chemistry.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curves A Level Chemistry The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong. Titration Curves A Level Chemistry.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curves A Level Chemistry Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. A titration curve can be used to determine. Titration Curves A Level Chemistry.
From www.youtube.com
Titration curves AS and ALevel chemistry YouTube Titration Curves A Level Chemistry Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. What are ph titration curves. Titration is a. Titration Curves A Level Chemistry.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curves A Level Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration is a technique used in neutralisation reactions between acids. Titration Curves A Level Chemistry.
From www.savemyexams.com
pH Titration Curves CIE A Level Chemistry Revision Notes 2025 Titration Curves A Level Chemistry It involves adding a titrant of known concentration. What are ph titration curves. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. The end point of a titration is the point at which an indicator changes. Without titles for the graph you can easily recognise which combination is shown. Titration Curves A Level Chemistry.
From www.savemyexams.com
pH Curves AQA A Level Chemistry Revision Notes 2017 Titration Curves A Level Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It. Titration Curves A Level Chemistry.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curves A Level Chemistry A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. A titration curve is a graphical. Titration Curves A Level Chemistry.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Titration Curves A Level Chemistry Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. Titration curves show how the ph of an. Titration Curves A Level Chemistry.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curves A Level Chemistry Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; A ph curve is a graphical representation that. Titration Curves A Level Chemistry.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curves A Level Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. A titration curve can be used to determine the pka or pkb of an unknown weak acid or base. It involves adding a titrant. Titration Curves A Level Chemistry.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curves A Level Chemistry What are ph titration curves. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. A ph curve. Titration Curves A Level Chemistry.
From www.savemyexams.com
pH Titration Curves OCR A Level Chemistry Revision Notes 2017 Titration Curves A Level Chemistry The end point of a titration is the point at which an indicator changes. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; A titration curve is a graphical representation of the ph of a solution during a titration. What are ph titration curves. Titration curves show how the. Titration Curves A Level Chemistry.
From www.hanlin.com
AQA A Level Chemistry复习笔记5.6.4 pH Curves翰林国际教育 Titration Curves A Level Chemistry Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an indicator changes. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Without. Titration Curves A Level Chemistry.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curves A Level Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. The end point of a titration is the point at which an. Titration Curves A Level Chemistry.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curves A Level Chemistry Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. A titration curve can be used. Titration Curves A Level Chemistry.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curves A Level Chemistry Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and deducing whether the acid and alkali are strong or weak. The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as. Titration Curves A Level Chemistry.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curves A Level Chemistry What are ph titration curves. It involves adding a titrant of known concentration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; A titration curve is. Titration Curves A Level Chemistry.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Titration Curves A Level Chemistry Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. What are ph titration curves. The end point of a titration is the point at which an indicator changes. Without. Titration Curves A Level Chemistry.
From www.savemyexams.com
pH Titration Curves CIE A Level Chemistry Revision Notes 2025 Titration Curves A Level Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Without titles for the graph you can easily recognise which combination is shown by looking at the starting and ending ph and. Titration Curves A Level Chemistry.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curves A Level Chemistry Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve can be used to determine the pka or pkb of an unknown weak acid. Titration Curves A Level Chemistry.
From www.youtube.com
how to draw AcidBase titration Curve and selection of Suitable Titration Curves A Level Chemistry Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution. Titration is a technique used in neutralisation reactions between acids and alkalis to determine the concentration of the unknown solution; A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how. Titration Curves A Level Chemistry.