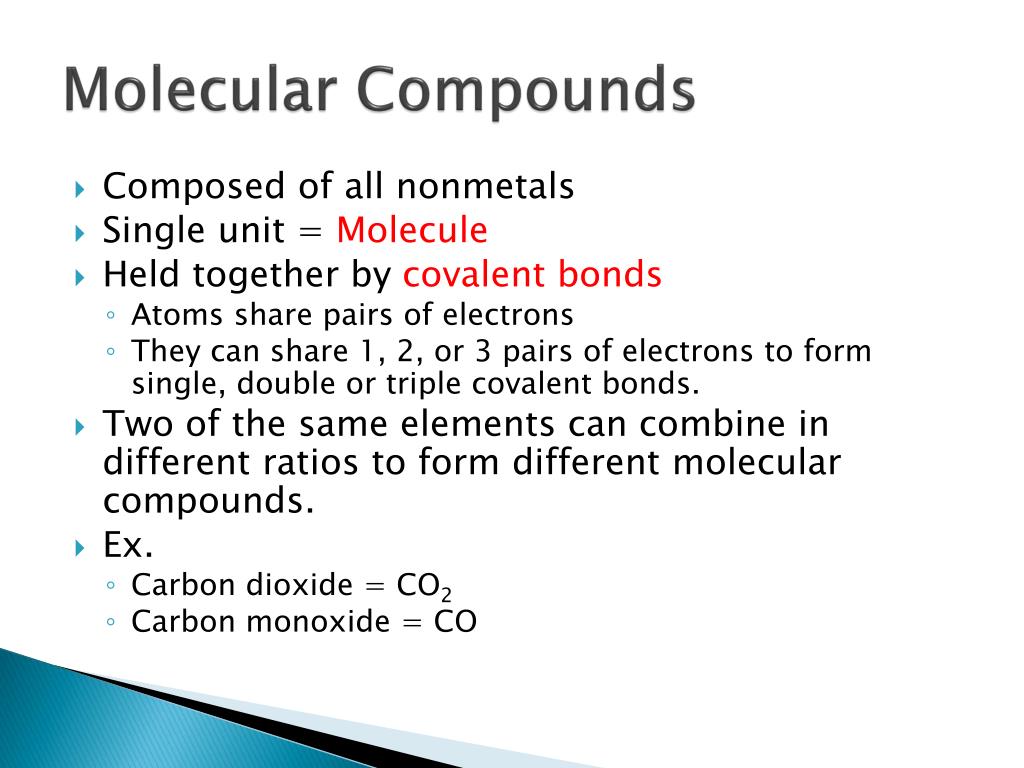
What Is True About Molecular Compounds . In lewis structures, element symbols represent atoms, and dots. Representations of molecular structure several methods of representing a molecule's structure. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Molecular compounds have lower melting points,. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Covalent or molecular compounds contain atoms held together by covalent bonds. These bonds form when the atoms share electrons because they have similar electronegativity values. Examples include such familiar substances as water (h2o) (h. Molecular compounds are chemical compounds that take the form of discrete molecules.
from www.slideserve.com
Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. Representations of molecular structure several methods of representing a molecule's structure. These bonds form when the atoms share electrons because they have similar electronegativity values. These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Examples include such familiar substances as water (h2o) (h. Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'.
PPT Molecular Compounds PowerPoint Presentation, free download ID
What Is True About Molecular Compounds Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. In lewis structures, element symbols represent atoms, and dots. These bonds form when the atoms share electrons because they have similar electronegativity values. Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Molecular compounds are chemical compounds that take the form of discrete molecules. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Molecular compounds have lower melting points,. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Representations of molecular structure several methods of representing a molecule's structure. These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Examples include such familiar substances as water (h2o) (h. Covalent or molecular compounds contain atoms held together by covalent bonds.
From studyingworksheets.com
Molecular Compounds Worksheet Studying Worksheets What Is True About Molecular Compounds Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Molecular compounds, sometimes called. What Is True About Molecular Compounds.
From thechemistrynotes.com
Molecular Compounds Important 2 Types, Properties, Uses What Is True About Molecular Compounds Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Molecular compounds are chemical compounds that take the form of discrete molecules. These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. These bonds form when the atoms share. What Is True About Molecular Compounds.
From ar.inspiredpencil.com
List Of Molecular Compounds What Is True About Molecular Compounds Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds have lower melting points,. In lewis structures, element symbols represent atoms, and dots. These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Just as an atom is the simplest unit. What Is True About Molecular Compounds.
From www.youtube.com
Formulas and Names of Molecular Compounds YouTube What Is True About Molecular Compounds Molecular compounds have lower melting points,. Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Just as an atom is the simplest unit that. What Is True About Molecular Compounds.
From www.slideserve.com
PPT CHEMISTRY The Central Science 9th Edition PowerPoint Presentation What Is True About Molecular Compounds Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. These atoms can be from the same element, like oxygen gas (o2), or different elements, such. What Is True About Molecular Compounds.
From sciencenotes.org
What Is a Molecule? Definition and Examples What Is True About Molecular Compounds These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Molecular compounds have lower melting points,. Examples include such familiar substances as water (h2o) (h. These bonds form when the atoms share electrons because they have similar electronegativity values. Molecular compounds are substances formed. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Section 4.2 Molecular Compounds PowerPoint Presentation, free What Is True About Molecular Compounds These bonds form when the atoms share electrons because they have similar electronegativity values. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. These atoms can be from the same element, like oxygen gas (o2),. What Is True About Molecular Compounds.
From www.youtube.com
Molecular Compounds YouTube What Is True About Molecular Compounds Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Examples include such familiar substances as water (h2o) (h. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Molecular compounds have lower melting points,. Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular. What Is True About Molecular Compounds.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples What Is True About Molecular Compounds Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds are chemical compounds that take the form of discrete molecules. These atoms can be from the same element, like oxygen gas. What Is True About Molecular Compounds.
From www.haikudeck.com
Molecular Compounds by Anton Pope What Is True About Molecular Compounds Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Examples include such familiar substances as water (h2o) (h. Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Representations of molecular structure several methods of representing a molecule's structure. Molecular compounds have lower melting points,. Molecular compounds, sometimes. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Naming Molecular Compounds PowerPoint Presentation, free download What Is True About Molecular Compounds Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Representations of molecular structure several methods of representing a molecule's structure. Molecular compounds have lower melting points,. In lewis structures, element symbols represent atoms, and dots. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Molecular compounds are chemical compounds. What Is True About Molecular Compounds.
From www.youtube.com
How to Name Molecular Compounds Examples and Practice Problems YouTube What Is True About Molecular Compounds Molecular compounds are substances formed when two or more atoms join together through chemical bonds. These bonds form when the atoms share electrons because they have similar electronegativity values. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. In lewis structures, element symbols represent atoms, and dots. Molecular compounds are. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download What Is True About Molecular Compounds Representations of molecular structure several methods of representing a molecule's structure. These bonds form when the atoms share electrons because they have similar electronegativity values. Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Molecular compounds have lower melting points,. Molecular compounds are chemical compounds that take the form of discrete molecules. Molecular. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID What Is True About Molecular Compounds Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Representations of molecular structure several methods of representing a molecule's structure. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. These atoms can be from the same element,. What Is True About Molecular Compounds.
From www.dreamstime.com
Common Chemical Compounds stock illustration. Illustration of molecule What Is True About Molecular Compounds Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Examples include such familiar substances as water (h2o) (h. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. These bonds form when the atoms share electrons because they have similar electronegativity values. Molecular compounds are. What Is True About Molecular Compounds.
From ar.inspiredpencil.com
List Of Molecular Compounds What Is True About Molecular Compounds Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Representations of molecular structure several methods of representing a molecule's structure. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. Covalent or molecular compounds contain atoms held. What Is True About Molecular Compounds.
From studylib.net
Nomenclature of Compounds What Is True About Molecular Compounds Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Representations of molecular structure several methods of representing a molecule's structure. Covalent or molecular compounds contain atoms held together by covalent bonds. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit. What Is True About Molecular Compounds.
From slidetodoc.com
Molecular Compounds What are molecular compounds and how What Is True About Molecular Compounds Molecular compounds have lower melting points,. These bonds form when the atoms share electrons because they have similar electronegativity values. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has. What Is True About Molecular Compounds.
From slideplayer.com
Molecular Compounds and their Covalent Bonds ppt download What Is True About Molecular Compounds These bonds form when the atoms share electrons because they have similar electronegativity values. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. In lewis structures, element symbols represent atoms, and dots. Examples include such. What Is True About Molecular Compounds.
From slidetodoc.com
Chapter 8 Molecular Compounds Molecular Compounds Covalent bondsAtoms What Is True About Molecular Compounds These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Molecular compounds are chemical. What Is True About Molecular Compounds.
From www.haikudeck.com
Molecular Compounds by Anton Pope What Is True About Molecular Compounds These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Molecular compounds are chemical compounds that take the form of discrete molecules. Covalent or molecular. What Is True About Molecular Compounds.
From slideplayer.com
Chapter 8 Molecular Compounds. ppt download What Is True About Molecular Compounds Molecular compounds have lower melting points,. These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Covalent compounds are a diverse group of molecules, so there are several exceptions. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download What Is True About Molecular Compounds In lewis structures, element symbols represent atoms, and dots. Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds have lower melting points,. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Molecular compounds are chemical compounds that take the form of discrete molecules. Molecular compounds are. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID What Is True About Molecular Compounds Molecular compounds are substances formed when two or more atoms join together through chemical bonds. In lewis structures, element symbols represent atoms, and dots. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. These atoms can be from the same element, like oxygen. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID What Is True About Molecular Compounds Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. In lewis structures, element symbols represent atoms, and. What Is True About Molecular Compounds.
From slideplayer.com
Chapter 8 Molecular Compounds ppt download What Is True About Molecular Compounds Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds are chemical compounds that take the form of discrete molecules. These bonds form when the atoms share electrons. What Is True About Molecular Compounds.
From examples.yourdictionary.com
Difference Between a Molecule and Compound Made Simple What Is True About Molecular Compounds Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds are chemical compounds that take the. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Chapter 2 Atoms, Molecules, and Ions PowerPoint Presentation What Is True About Molecular Compounds Representations of molecular structure several methods of representing a molecule's structure. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Molecular compounds are substances. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation ID2672985 What Is True About Molecular Compounds These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Molecular compounds are substances formed when two or more atoms join together through chemical bonds. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of.. What Is True About Molecular Compounds.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID What Is True About Molecular Compounds Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has. What Is True About Molecular Compounds.
From www.slideserve.com
PPT 4.2 Representing Molecular Compounds Part I PowerPoint What Is True About Molecular Compounds Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the. Covalent or molecular compounds contain atoms held together by covalent bonds. Representations of molecular structure several methods of representing a. What Is True About Molecular Compounds.
From www.youtube.com
How to Name and Write Formulas of Molecular Compounds YouTube What Is True About Molecular Compounds Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. Molecular compounds are chemical compounds that take the form of discrete molecules. Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds have lower melting points,. In lewis. What Is True About Molecular Compounds.
From www.slideshare.net
Molecular Compounds What Is True About Molecular Compounds Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds have lower melting points,. Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. Molecular compounds, sometimes called covalent compounds, display a wide range of physical properties due to the different types of. Examples include such familiar substances as water (h2o) (h. Representations. What Is True About Molecular Compounds.
From chem.libretexts.org
2.6 Molecules and Molecular Compounds Chemistry LibreTexts What Is True About Molecular Compounds These atoms can be from the same element, like oxygen gas (o2), or different elements, such as water (h2o), where hydrogen and oxygen atoms bond together. Covalent or molecular compounds contain atoms held together by covalent bonds. Molecular compounds have lower melting points,. Molecular compounds are chemical compounds that take the form of discrete molecules. Examples include such familiar substances. What Is True About Molecular Compounds.
From slideplayer.com
Binary Molecular Compounds ppt download What Is True About Molecular Compounds Covalent compounds are a diverse group of molecules, so there are several exceptions to each 'rule'. Molecular compounds are made up of neutral, nonmetal atoms that share electrons in covalent bonds. In lewis structures, element symbols represent atoms, and dots. Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is. What Is True About Molecular Compounds.