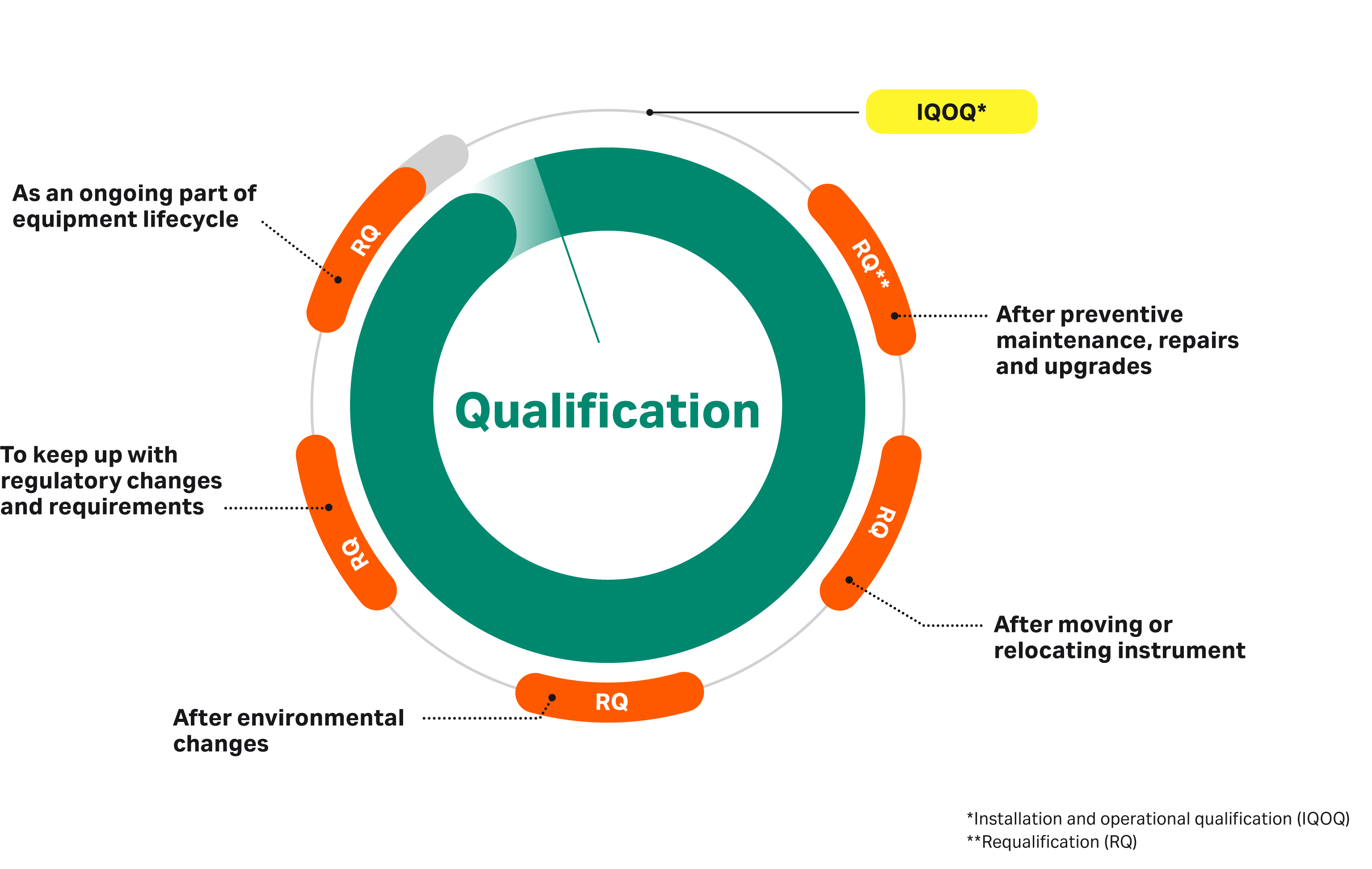
Lab Equipment Qualification . We now address what has changed in the new version of usp. Approach for carrying out an analytical instrument qualification (aiq). The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. To audit vendor accountability, it is crucial to. Detailed instrument operating parameters to be qualified are found in the. There are four critical components involved in the generation of reliable and consistent analytical. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. The operational qualification (oq) section—a small but significant change. Manual 053 laboratory equipment qualification. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its.
from www.cytivalifesciences.com.cn
Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Detailed instrument operating parameters to be qualified are found in the. To audit vendor accountability, it is crucial to. We now address what has changed in the new version of usp. Manual 053 laboratory equipment qualification. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. The operational qualification (oq) section—a small but significant change.
Stay compliant instrument qualification for cGMP Cytiva
Lab Equipment Qualification Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. The operational qualification (oq) section—a small but significant change. There are four critical components involved in the generation of reliable and consistent analytical. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. To audit vendor accountability, it is crucial to. We now address what has changed in the new version of usp. Manual 053 laboratory equipment qualification. Detailed instrument operating parameters to be qualified are found in the. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. Approach for carrying out an analytical instrument qualification (aiq).
From www.aplyon.com
Installation Qualification IQ Procedure Lab Equipment Qualification Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. There are four critical components involved in the generation of reliable and consistent analytical. To audit vendor accountability, it is crucial to. Detailed instrument operating parameters to be qualified are found in the. The operational qualification (oq) section—a small but significant change.. Lab Equipment Qualification.
From clipartmag.com
Science Equipment Drawings Free download on ClipArtMag Lab Equipment Qualification The operational qualification (oq) section—a small but significant change. Manual 053 laboratory equipment qualification. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. To audit vendor accountability, it is crucial to. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. When introducing new. Lab Equipment Qualification.
From giodurnhd.blob.core.windows.net
Equipment Qualification Protocol Template at Thelma Robertson blog Lab Equipment Qualification To audit vendor accountability, it is crucial to. Manual 053 laboratory equipment qualification. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Good laboratory practice (glp) regulations are appropriate resources. Lab Equipment Qualification.
From studylib.net
Lab Regulations MI CLIA Policy Lab Equipment Qualification The operational qualification (oq) section—a small but significant change. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Manual 053 laboratory equipment qualification. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Approach for carrying out an analytical instrument qualification (aiq). Thus,. Lab Equipment Qualification.
From www.careofair.com
Laboratory equipment qualification and validation Lab Equipment Qualification We now address what has changed in the new version of usp. Manual 053 laboratory equipment qualification. To audit vendor accountability, it is crucial to. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. Detailed instrument operating parameters to be qualified are found in the. Good laboratory practice. Lab Equipment Qualification.
From kvalito.ch
Commissioning, Qualification, and Validation (CQV) for Facility Lab Equipment Qualification The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Manual 053 laboratory equipment qualification. Detailed instrument. Lab Equipment Qualification.
From www.youtube.com
Equipment Qualification in Clinical Lab Part III Performance Lab Equipment Qualification Approach for carrying out an analytical instrument qualification (aiq). The operational qualification (oq) section—a small but significant change. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. We now address what has changed in the new version of usp. Manual 053 laboratory equipment qualification. Ensuring laboratory equipment operates as expected is. Lab Equipment Qualification.
From www.careofair.com
Laboratory equipment qualification and validation Lab Equipment Qualification Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Detailed instrument operating parameters to be qualified are found in the. There are four critical components involved in the generation of reliable and consistent analytical. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with. Lab Equipment Qualification.
From www.theoverbrookgroup.com
HPLC Qualification (IQ/OQ/PQ) Ensuring Your Data Integrity Lab Equipment Qualification Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. The operational qualification (oq) section—a small but significant change. Detailed instrument operating parameters to be qualified are found in the. Approach for carrying out an analytical instrument qualification (aiq). Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment. Lab Equipment Qualification.
From fullspectrumlabservices.com
Lab Equipment Qualification Services Full Spectrum Lab Services from CBRE Lab Equipment Qualification Manual 053 laboratory equipment qualification. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. There are four critical components involved in the generation of reliable and consistent analytical. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g.. Lab Equipment Qualification.
From qbdgroup.com
Qualification of laboratory equipment key considerations Lab Equipment Qualification The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. To audit vendor accountability, it is crucial to. There are four critical components involved in the generation of reliable and consistent analytical. We now address what has changed in the new version of usp. When introducing new laboratory equipment for analytical testing. Lab Equipment Qualification.
From www.yumpu.com
Laboratory Equipment Qualification Pharmaceutical Technology Lab Equipment Qualification Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. Good. Lab Equipment Qualification.
From www.youtube.com
Equipment Qualification YouTube Lab Equipment Qualification Manual 053 laboratory equipment qualification. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. The operational qualification (oq) section—a small but significant change. Good laboratory practice (glp) regulations are appropriate resources. Lab Equipment Qualification.
From www.researchgate.net
Flow Sheet of the Qualification Procedure. Download Scientific Diagram Lab Equipment Qualification There are four critical components involved in the generation of reliable and consistent analytical. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. The operational qualification (oq) section—a small but significant. Lab Equipment Qualification.
From pharmsolution.org
Laboratory instrument qualification Pharma Solutions Ltd Lab Equipment Qualification Manual 053 laboratory equipment qualification. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. To audit vendor accountability, it is crucial to. Approach for carrying out an analytical instrument qualification. Lab Equipment Qualification.
From www.hamiltoncompany.com
Qualification (IQ/OQ) Support Process Analytics Hamilton Company Lab Equipment Qualification Approach for carrying out an analytical instrument qualification (aiq). We now address what has changed in the new version of usp. There are four critical components involved in the generation of reliable and consistent analytical. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. The current usp approach is. Lab Equipment Qualification.
From ch.linkedin.com
Alina Saliji QC Lab Equipment Qualification Specialist The Janssen Lab Equipment Qualification The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. To audit vendor accountability, it is crucial to. Detailed instrument operating parameters to be qualified are found in the. Ensuring laboratory equipment. Lab Equipment Qualification.
From pharmsolution.org
Qualification Pharma Solutions Ltd Lab Equipment Qualification Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. To audit vendor accountability, it is crucial to. Ensuring laboratory equipment operates as expected is critically important to the validity of results. Lab Equipment Qualification.
From www.cytivalifesciences.com.cn
Stay compliant instrument qualification for cGMP Cytiva Lab Equipment Qualification When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. Detailed instrument operating parameters to be qualified are found in the. The operational qualification (oq) section—a small but significant change. There are four. Lab Equipment Qualification.
From mpslab.ba
Calibration and qualification of equipment MPS Lab Lab Equipment Qualification Manual 053 laboratory equipment qualification. Detailed instrument operating parameters to be qualified are found in the. There are four critical components involved in the generation of reliable and consistent analytical. Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. The operational qualification (oq) section—a small but significant change.. Lab Equipment Qualification.
From issuu.com
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd Issuu Lab Equipment Qualification When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. Detailed instrument operating parameters to be qualified are found in the. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. The operational qualification (oq) section—a small but significant change. To audit vendor. Lab Equipment Qualification.
From www.nalys-group.com
V cycle in the pharmaceutical environment Nalys Lab Equipment Qualification To audit vendor accountability, it is crucial to. There are four critical components involved in the generation of reliable and consistent analytical. The operational qualification (oq) section—a small but significant change. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. Detailed instrument operating parameters to be qualified are found in the. Ensuring. Lab Equipment Qualification.
From www.studocu.com
Microbiology Equipments ( Overview) MICROBIOLOGY LABORATORY EQUIPMENT Lab Equipment Qualification Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. We now address what has changed in the new version of usp. The operational qualification (oq) section—a small but significant change. The. Lab Equipment Qualification.
From www.studocu.com
Mpharmasyl 1718139141 134 3 Qualification of laboratory equipments Lab Equipment Qualification We now address what has changed in the new version of usp. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. To audit vendor accountability, it is crucial to. Detailed instrument operating parameters to be qualified are found in the. Good laboratory practice (glp) regulations are appropriate resources to. Lab Equipment Qualification.
From loeevsykv.blob.core.windows.net
Validation New Laboratory Equipment at Calvin Fischer blog Lab Equipment Qualification Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. To audit vendor accountability, it is crucial to. Manual 053 laboratory equipment qualification. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. Ensuring laboratory equipment operates as expected. Lab Equipment Qualification.
From www.congress-intercultural.eu
IQ OQ PQ Templates Download Professional Templates, 41 OFF Lab Equipment Qualification Detailed instrument operating parameters to be qualified are found in the. There are four critical components involved in the generation of reliable and consistent analytical. To audit vendor accountability, it is crucial to. The operational qualification (oq) section—a small but significant change. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various. Lab Equipment Qualification.
From www.getreskilled.com
What's an IQ OQ PQ Validation Protocol & why's it critical in pharma? Lab Equipment Qualification Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. The operational qualification (oq) section—a small but significant change. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. The current usp approach is to qualify the instrument for the expected. Lab Equipment Qualification.
From www.careofair.com
Laboratory equipment qualification and validation Lab Equipment Qualification There are four critical components involved in the generation of reliable and consistent analytical. We now address what has changed in the new version of usp. When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. Manual 053 laboratory equipment qualification. Approach for carrying out an analytical instrument qualification (aiq).. Lab Equipment Qualification.
From www.getreskilled.com
Commissioning vs Qualification vs Validation in Pharma GetReskilled Lab Equipment Qualification The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Detailed instrument operating parameters to be qualified are found in the. Approach for carrying out an analytical instrument qualification (aiq). Manual 053 laboratory equipment qualification. There are four critical components involved in the generation of reliable and consistent analytical. The operational qualification. Lab Equipment Qualification.
From portal.rsisjs.id
PORTAL RSI SURABAYA JEMURSARI Lab Equipment Qualification Manual 053 laboratory equipment qualification. We now address what has changed in the new version of usp. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. When introducing new laboratory equipment for analytical testing. Lab Equipment Qualification.
From www.studypool.com
SOLUTION Activity 2 Laboratory Equipment Used in Microbiology Lab Equipment Qualification Thus, the phrase analytical instrument qualification (aiq) is used for the process of ensuring that an instrument is suitable for its. To audit vendor accountability, it is crucial to. We now address what has changed in the new version of usp. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. When. Lab Equipment Qualification.
From www.skillpad.com
Laboratory Equipment Qualification Skillpad Lab Equipment Qualification The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. Ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. To audit vendor accountability, it is crucial. Lab Equipment Qualification.
From studylib.net
Laboratory Instrument Qualification Solving the Puzzle Equipment Lab Equipment Qualification When introducing new laboratory equipment for analytical testing of gmp products, qualification is mandatory to comply with various regulations (e.g. Approach for carrying out an analytical instrument qualification (aiq). To audit vendor accountability, it is crucial to. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. There are four critical components involved. Lab Equipment Qualification.
From www.inpaspages.com
Equipment test records Lab Equipment Qualification Detailed instrument operating parameters to be qualified are found in the. To audit vendor accountability, it is crucial to. Good laboratory practice (glp) regulations are appropriate resources to provide guidance on equipment design, calibration, and maintenance. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Ensuring laboratory equipment operates as expected. Lab Equipment Qualification.
From www.slideshare.net
1 5 equipmentqualification Lab Equipment Qualification Detailed instrument operating parameters to be qualified are found in the. To audit vendor accountability, it is crucial to. There are four critical components involved in the generation of reliable and consistent analytical. The current usp approach is to qualify the instrument for the expected operating range, thereby implicitly verifying the. Thus, the phrase analytical instrument qualification (aiq) is used. Lab Equipment Qualification.