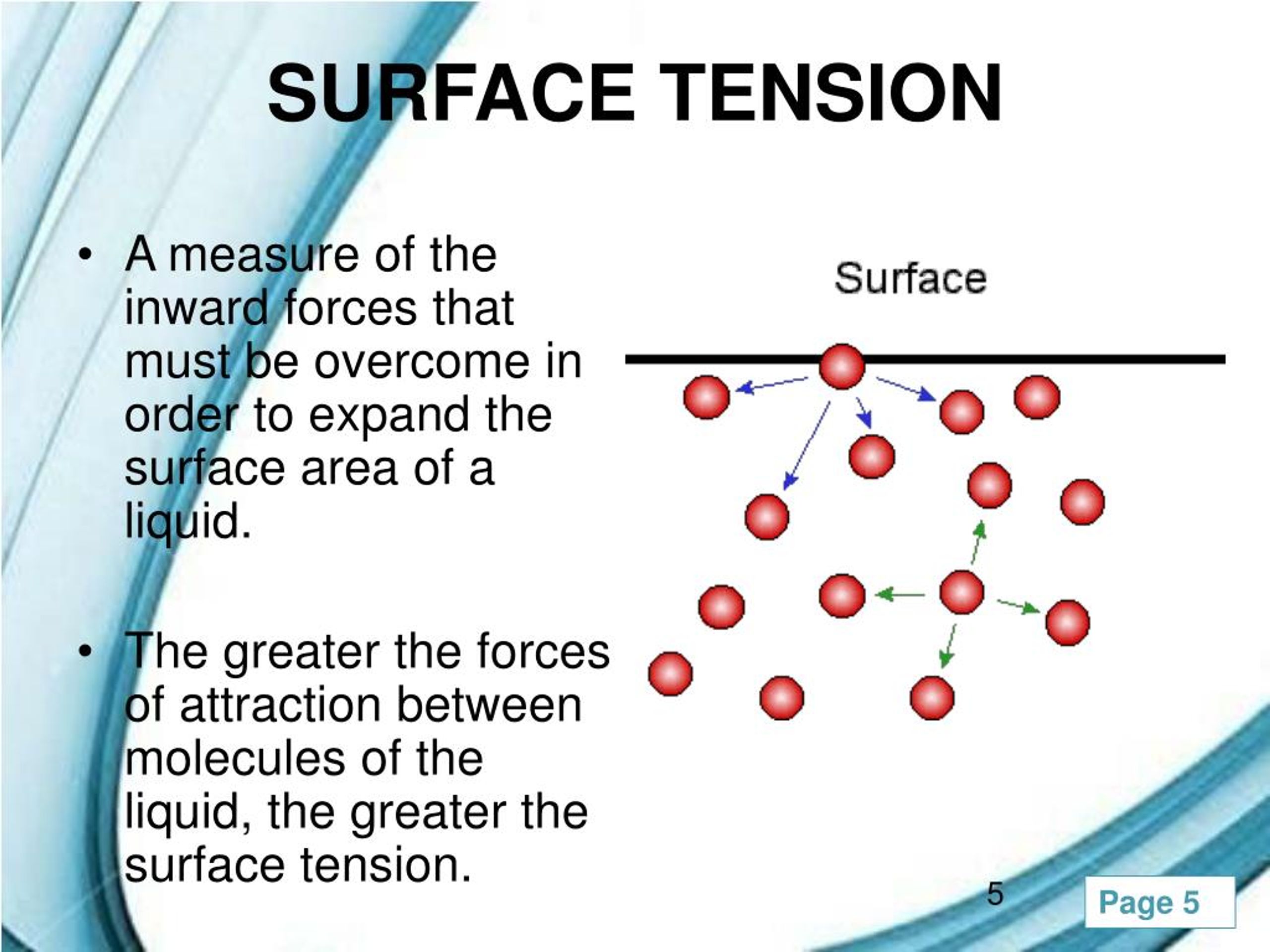
What Is A High Surface Tension Liquid . This term is typically used only. The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Since these intermolecular forces vary. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. A fluid surface has a. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This phenomenon can be observed in the nearly. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension.
from www.slideserve.com
Since these intermolecular forces vary. This term is typically used only. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. This phenomenon can be observed in the nearly. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. A fluid surface has a. The molecules at the surface do not have other like molecules on all sides of them.
PPT Properties of Liquids and Solids PowerPoint Presentation, free
What Is A High Surface Tension Liquid A fluid surface has a. Since these intermolecular forces vary. The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. A fluid surface has a. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. This phenomenon can be observed in the nearly. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This term is typically used only.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Is A High Surface Tension Liquid Since these intermolecular forces vary. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules at the surface do not have. What Is A High Surface Tension Liquid.
From read.cholonautas.edu.pe
Why Water Has High Surface Tension Printable Templates Free What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This term. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is a physical property defined as the amount of force required per unit area to. What Is A High Surface Tension Liquid.
From www.scienceabc.com
Why Does Water Feel Like Concrete When You Belly Flop Into It? » ScienceABC What Is A High Surface Tension Liquid This phenomenon can be observed in the nearly. This term is typically used only. The molecules at the surface do not have other like molecules on all sides of them. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Simply put, surface tension is the tendency of molecules of a. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension is a physical. What Is A High Surface Tension Liquid.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Is A High Surface Tension Liquid Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. A fluid surface has a. The molecules at the surface do not have other like molecules on all sides of them. The cohesive forces between liquid molecules are responsible for the phenomenon known. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID9168408 What Is A High Surface Tension Liquid A fluid surface has a. This term is typically used only. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. The molecules at the surface do not have other like molecules on all sides of them. Surface tension, property of a liquid surface displayed by. What Is A High Surface Tension Liquid.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. A fluid surface has a. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. What Is A High Surface Tension Liquid.
From smartquizbasket.blogspot.com
Smart Quiz Basket Surface Tension Definition Chemistry What Is A High Surface Tension Liquid This term is typically used only. A fluid surface has a. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin. What Is A High Surface Tension Liquid.
From www.youtube.com
Surface Tension of Water Explained YouTube What Is A High Surface Tension Liquid Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. This phenomenon can be observed in the nearly. Surface tension is the energy, or work, required to increase. What Is A High Surface Tension Liquid.
From ar.inspiredpencil.com
Surface Tension Water What Is A High Surface Tension Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The cohesive forces between liquid molecules are responsible for the phenomenon known as. What Is A High Surface Tension Liquid.
From www.thoughtco.com
Surface Tension Definition in Chemistry What Is A High Surface Tension Liquid A fluid surface has a. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This term is typically used only. Since these intermolecular forces vary. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface do not have other like. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Properties of Liquids and Solids PowerPoint Presentation, free What Is A High Surface Tension Liquid The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is. What Is A High Surface Tension Liquid.
From ar.inspiredpencil.com
Surface Tension Water What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. Since these intermolecular forces vary. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only. Surface tension is a physical property. What Is A High Surface Tension Liquid.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co What Is A High Surface Tension Liquid This phenomenon can be observed in the nearly. This term is typically used only. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is a physical property defined as the amount of force required per unit area to expand the. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Forces of attraction/repulsion between molecules. PowerPoint What Is A High Surface Tension Liquid A fluid surface has a. The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. This. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free What Is A High Surface Tension Liquid A fluid surface has a. The molecules at the surface do not have other like molecules on all sides of them. Since these intermolecular forces vary. This phenomenon can be observed in the nearly. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation What Is A High Surface Tension Liquid Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This phenomenon can be observed in the nearly. This term is typically used only. The molecules at the surface do not have other like molecules on all sides of them. The cohesive forces between liquid. What Is A High Surface Tension Liquid.
From almerja.com
Surface Tension What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. This phenomenon can be observed in the nearly. This term is typically used only. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension, property of a liquid surface displayed by its acting as if it were. What Is A High Surface Tension Liquid.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Is A High Surface Tension Liquid A fluid surface has a. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. Surface tension is a phenomenon in which. What Is A High Surface Tension Liquid.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b What Is A High Surface Tension Liquid This phenomenon can be observed in the nearly. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. Since these intermolecular forces vary. A fluid surface has a. This term is typically used only. The molecules at the surface do not have other like molecules on. What Is A High Surface Tension Liquid.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Is A High Surface Tension Liquid This term is typically used only. Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a phenomenon in which the surface of a. What Is A High Surface Tension Liquid.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Is A High Surface Tension Liquid Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. A fluid surface has a. The molecules at the surface do not have other like molecules on all sides of them. This term is typically used only. Surface tension, property of a liquid surface displayed by its. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 What Is A High Surface Tension Liquid Since these intermolecular forces vary. This term is typically used only. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a physical property defined as the amount of force required. What Is A High Surface Tension Liquid.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Surface tension is a physical property defined as the amount of force required per unit area to. What Is A High Surface Tension Liquid.
From byjus.com
Explain the surface tension phenomenon with examples. What Is A High Surface Tension Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. Since these intermolecular forces vary. A fluid surface has a. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This term is typically used only.. What Is A High Surface Tension Liquid.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids What Is A High Surface Tension Liquid This phenomenon can be observed in the nearly. The molecules at the surface do not have other like molecules on all sides of them. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, property of a liquid surface displayed by its acting as if it were a. What Is A High Surface Tension Liquid.
From www.dataphysics-instruments.com
Interfacial and surface tension of liquids explained DataPhysics What Is A High Surface Tension Liquid Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. The molecules at the surface do not have other like molecules on all sides of them. This phenomenon can be observed in the nearly. A fluid surface has a. This term is typically used only.. What Is A High Surface Tension Liquid.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Is A High Surface Tension Liquid Surface tension is a physical property defined as the amount of force required per unit area to expand the surface of a liquid. The molecules at the surface do not have other like molecules on all sides of them. Since these intermolecular forces vary. This phenomenon can be observed in the nearly. Surface tension is a phenomenon in which the. What Is A High Surface Tension Liquid.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download What Is A High Surface Tension Liquid A fluid surface has a. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. Surface tension is a physical property defined as the amount of force required per unit. What Is A High Surface Tension Liquid.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Is A High Surface Tension Liquid The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. This phenomenon can be observed in the nearly. Since these intermolecular forces vary. The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a physical property defined as the amount of force required per unit area. What Is A High Surface Tension Liquid.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with What Is A High Surface Tension Liquid Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. This term. What Is A High Surface Tension Liquid.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a. What Is A High Surface Tension Liquid.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Is A High Surface Tension Liquid The molecules at the surface do not have other like molecules on all sides of them. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a. What Is A High Surface Tension Liquid.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Is A High Surface Tension Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a. This term is typically used only. Since these intermolecular forces vary. The molecules at the surface do not have other like molecules on all sides of them. Surface tension is a phenomenon in which the surface. What Is A High Surface Tension Liquid.