Potassium Iodide Solubility In Oil . The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. With moisture present, ki forms a. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. It’s colorless in its pure form and has a distinctive, sharp, salty taste. Potassium iodide appears as white, crystalline granules or powder. The parameter db/dt was used as a criterion for the evaluation. This monograph for potassium iodide provides, in addition to common physical constants, a general description including.
from www.bartleby.com
Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. Potassium iodide appears as white, crystalline granules or powder. It’s colorless in its pure form and has a distinctive, sharp, salty taste. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. With moisture present, ki forms a. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. The parameter db/dt was used as a criterion for the evaluation.
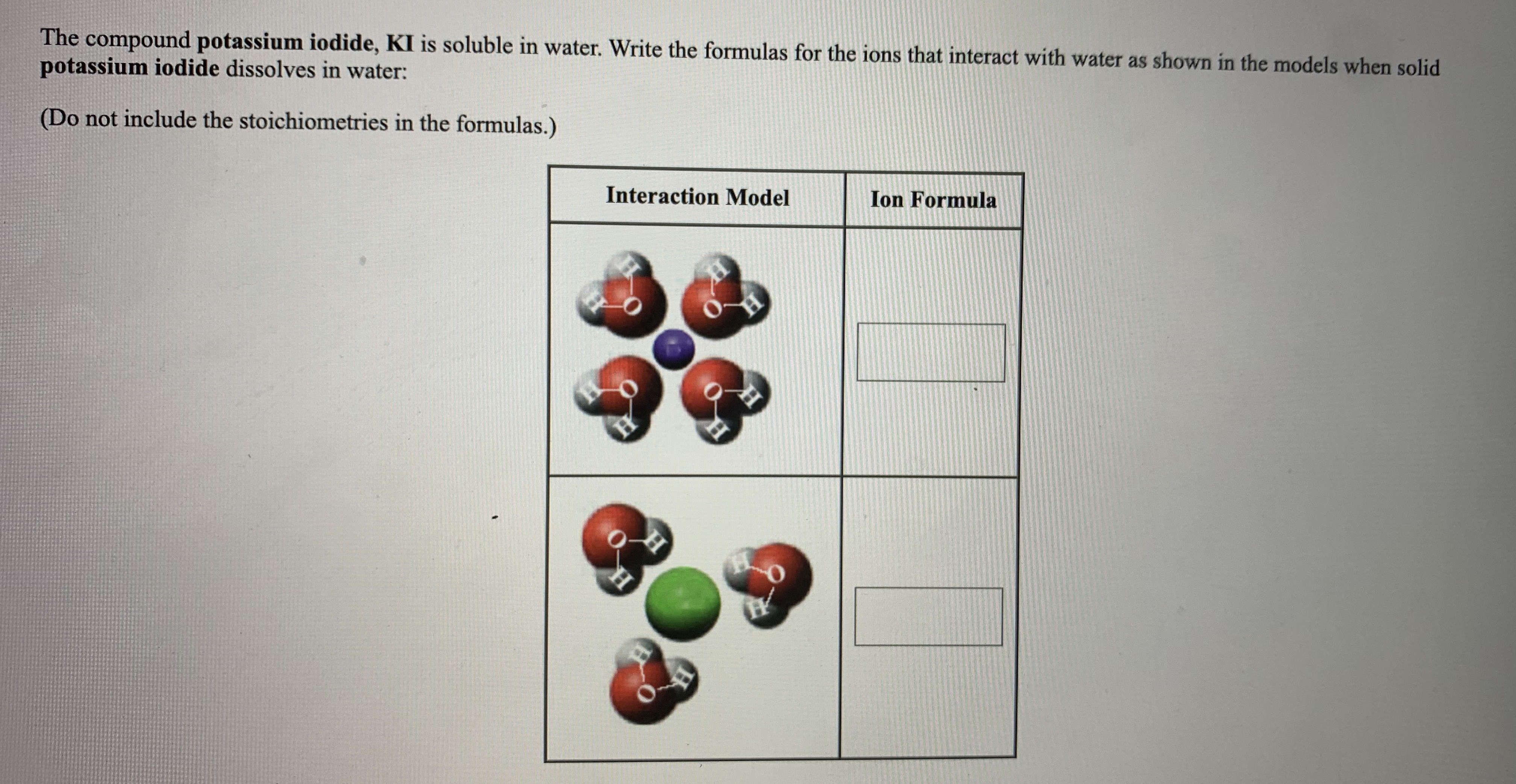
Answered The compound potassium iodide, KI is… bartleby
Potassium Iodide Solubility In Oil With moisture present, ki forms a. It’s colorless in its pure form and has a distinctive, sharp, salty taste. Potassium iodide appears as white, crystalline granules or powder. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. The parameter db/dt was used as a criterion for the evaluation. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. With moisture present, ki forms a. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes.
From www.sciencephoto.com
Potassium iodide solution Stock Image C043/2650 Science Photo Library Potassium Iodide Solubility In Oil This monograph for potassium iodide provides, in addition to common physical constants, a general description including. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Potassium iodide appears as white, crystalline granules or powder. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. The parameter db/dt. Potassium Iodide Solubility In Oil.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. It’s colorless in its pure form and has. Potassium Iodide Solubility In Oil.
From www.chemix-chemistry-software.com
Solubility Curves Potassium Iodide Solubility In Oil With moisture present, ki forms a. Potassium iodide appears as white, crystalline granules or powder. The parameter db/dt was used as a criterion for the evaluation. It’s colorless in its pure form and has a distinctive, sharp, salty taste. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. Skin contact of potassium iodide. Potassium Iodide Solubility In Oil.
From socratic.org
Question 71458 Socratic Potassium Iodide Solubility In Oil It’s colorless in its pure form and has a distinctive, sharp, salty taste. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. With moisture present, ki forms a. Potassium iodide appears. Potassium Iodide Solubility In Oil.
From www.fishersci.com
Potassium Iodide, 25 (w/v) Solution, Spectrum Chemical, Quantity 1 L Potassium Iodide Solubility In Oil Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Potassium iodide appears as white, crystalline granules or powder. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. The parameter db/dt was used as a criterion for the evaluation. Light and moisture accelerate the decomposition. Potassium Iodide Solubility In Oil.
From www.researchgate.net
Solubility of potassium sulfate in water and its predicted Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. Potassium iodide appears as white, crystalline granules or powder. The parameter db/dt was used as a criterion for. Potassium Iodide Solubility In Oil.
From www.academia.edu
(PDF) Solubility and Density of Potassium Iodide in Binary Ethanol Potassium Iodide Solubility In Oil It’s colorless in its pure form and has a distinctive, sharp, salty taste. With moisture present, ki forms a. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. Potassium iodide appears as white, crystalline granules or powder. Skin contact of potassium iodide may cause drying of the. Potassium Iodide Solubility In Oil.
From www.scribd.com
COA Potassium Iodide PDF Potassium Iodide Solubility In Oil Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. It’s colorless in its pure form and has a distinctive, sharp, salty taste. The parameter db/dt was used as a criterion for the evaluation. This monograph for potassium. Potassium Iodide Solubility In Oil.
From brainly.com
Use the solubility curve above to answer the following If I add 130 g Potassium Iodide Solubility In Oil The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. Potassium iodide appears as white, crystalline granules or powder. With moisture present, ki forms a. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. The parameter db/dt. Potassium Iodide Solubility In Oil.
From www.thoughtco.com
Precipitation Reaction Using Solubility Rules Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. The parameter db/dt was used as a criterion for the evaluation. With moisture present, ki forms a. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. This. Potassium Iodide Solubility In Oil.
From kyleighqwsolomon.blogspot.com
potassium iodide conductivity Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. The parameter db/dt was used as a criterion for the evaluation. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. Potassium iodide appears as white, crystalline granules or powder. With moisture present, ki. Potassium Iodide Solubility In Oil.
From www.nagwa.com
Question Video Using the Water Solubility Rules to Determine Which Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. It’s colorless in its pure form and has a distinctive, sharp, salty taste. Potassium iodide appears as white, crystalline granules or powder. This monograph for potassium. Potassium Iodide Solubility In Oil.
From www.slideserve.com
PPT Reactions in Solution PowerPoint Presentation, free download ID Potassium Iodide Solubility In Oil It’s colorless in its pure form and has a distinctive, sharp, salty taste. The parameter db/dt was used as a criterion for the evaluation. Potassium iodide appears as white, crystalline granules or powder. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. With moisture present, ki forms a. This monograph for. Potassium Iodide Solubility In Oil.
From www.numerade.com
Potassium iodide can be the additive in "iodized" table salt. Calculate Potassium Iodide Solubility In Oil It’s colorless in its pure form and has a distinctive, sharp, salty taste. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. The parameter db/dt was used as a criterion for the evaluation. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. Potassium iodide. Potassium Iodide Solubility In Oil.
From www.youtube.com
Electrolysis of concentrated potassium iodide YouTube Potassium Iodide Solubility In Oil Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. With moisture present, ki forms a. It’s colorless in its pure form and has a distinctive, sharp, salty taste. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Potassium iodide appears as white, crystalline granules or powder.. Potassium Iodide Solubility In Oil.
From www.researchgate.net
Temperature dependences of iodine solubility in (1) water and (2) 0.1 Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. It’s colorless in its pure form and has a distinctive, sharp, salty taste. With moisture present, ki forms a. Reaction with sodium. Potassium Iodide Solubility In Oil.
From www.pragmetis.com
Potassium Iodide Applications & Uses, and Warnings Pragmetis Potassium Iodide Solubility In Oil The parameter db/dt was used as a criterion for the evaluation. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. Potassium iodide appears as white, crystalline granules or powder. With moisture present, ki forms a. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes.. Potassium Iodide Solubility In Oil.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Potassium Iodide Solubility In Oil It’s colorless in its pure form and has a distinctive, sharp, salty taste. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. With moisture present, ki forms a. The solutions of. Potassium Iodide Solubility In Oil.
From www.bartleby.com
Answered The compound potassium iodide, KI is… bartleby Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. It’s colorless in its pure form and has a distinctive, sharp, salty taste. With moisture present, ki forms a. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Reaction with sodium. Potassium Iodide Solubility In Oil.
From www.numerade.com
SOLVED Solid potassium iodide into iodine gas and solid Potassium Iodide Solubility In Oil Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. It’s colorless in its pure form and has a distinctive, sharp, salty taste. The parameter db/dt was used as a criterion for the evaluation. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. Potassium iodide. Potassium Iodide Solubility In Oil.
From dokumen.tips
(PDF) Solubility and Density of Potassium Iodide in a Binary Propan1 Potassium Iodide Solubility In Oil With moisture present, ki forms a. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. Potassium iodide appears as white, crystalline granules or powder. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. It’s colorless in its pure. Potassium Iodide Solubility In Oil.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Potassium Iodide Potassium Iodide Solubility In Oil The parameter db/dt was used as a criterion for the evaluation. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. It’s colorless in its pure form and has a distinctive, sharp, salty taste. Potassium iodide appears as white, crystalline granules or powder. The solutions of potassium iodide in oil + dmf solvent are. Potassium Iodide Solubility In Oil.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Potassium Iodide Solubility In Oil Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. With moisture present, ki forms a. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. Light and moisture accelerate the decomposition. Potassium Iodide Solubility In Oil.
From www.numerade.com
SOLVED "solid potassium iodide into iodine gas and solid Potassium Iodide Solubility In Oil Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. Potassium iodide appears as white, crystalline granules or powder. The parameter db/dt was used as a criterion for the evaluation. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. The solutions of potassium iodide in. Potassium Iodide Solubility In Oil.
From www.numerade.com
SOLVED System 6 Double Replacement Reactions lead(I) nitrate and Potassium Iodide Solubility In Oil It’s colorless in its pure form and has a distinctive, sharp, salty taste. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. With moisture present, ki forms a. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. Skin contact of potassium iodide. Potassium Iodide Solubility In Oil.
From brainly.com
1. What is the solubility of potassium nitrate at 90°C. 2. What is the Potassium Iodide Solubility In Oil Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. It’s colorless in its pure form and has a distinctive, sharp, salty taste. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. With moisture present, ki forms a. Skin contact of potassium iodide may cause drying of the skin. Potassium Iodide Solubility In Oil.
From socratic.org
How would you determine the solubility of potassium nitrate? Socratic Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. The parameter db/dt was used as a criterion for the evaluation. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. Skin contact of potassium iodide may cause drying of the skin due to. Potassium Iodide Solubility In Oil.
From en.wikipedia.org
Potassium iodide Wikipedia Potassium Iodide Solubility In Oil With moisture present, ki forms a. The parameter db/dt was used as a criterion for the evaluation. Potassium iodide appears as white, crystalline granules or powder. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. This monograph for potassium iodide provides, in addition to common physical constants, a general description including.. Potassium Iodide Solubility In Oil.
From www.engineeringtoolbox.com
Densities of Aqueous Solutions of Potassium Salts Potassium Iodide Solubility In Oil Potassium iodide appears as white, crystalline granules or powder. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble in ammonium. The parameter db/dt was used as a criterion for the evaluation. It’s colorless in its. Potassium Iodide Solubility In Oil.
From www.numerade.com
SOLVEDAmmonia and potassium iodide solutions were added to an aqueous Potassium Iodide Solubility In Oil Potassium iodide appears as white, crystalline granules or powder. With moisture present, ki forms a. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. The parameter db/dt was used as a criterion for the evaluation. It’s colorless in its pure form and has a distinctive, sharp, salty. Potassium Iodide Solubility In Oil.
From brainly.com
Which compound demonstrated a constant rate of increase of solubility Potassium Iodide Solubility In Oil With moisture present, ki forms a. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. This monograph for potassium iodide provides, in addition to common physical constants, a general description including. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and. Potassium Iodide Solubility In Oil.
From www.numerade.com
SOLVED Salt Solubility at 30°C 60°C POTASSIUM IODIDE POTASSIUM Potassium Iodide Solubility In Oil With moisture present, ki forms a. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. Skin contact of potassium iodide may cause drying of the skin due to absorption of oils and moisture. This monograph for potassium iodide provides, in addition to common physical constants, a general description including.. Potassium Iodide Solubility In Oil.
From www.slideserve.com
PPT Chapter 4 Chemical Reactions An Introduction PowerPoint Potassium Iodide Solubility In Oil The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. With moisture present, ki forms a. Potassium iodide appears as white, crystalline granules or powder. It’s colorless in its pure form and has a distinctive, sharp, salty taste. Light and moisture accelerate the decomposition of this compound, and. Potassium Iodide Solubility In Oil.
From www.numerade.com
SOLVED 1). When aqueous solutions of potassium phosphate and chromium Potassium Iodide Solubility In Oil Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. The solutions of potassium iodide in oil + dmf solvent are newtonian behavior fluids, because they are true solutions and exhibit a. The parameter db/dt was used as a criterion for the evaluation. Reaction with sodium bitartrate produces a white. Potassium Iodide Solubility In Oil.
From www.semanticscholar.org
Figure 4 from Solubility and Density of Silver Iodide in Water and DMF Potassium Iodide Solubility In Oil Potassium iodide appears as white, crystalline granules or powder. Light and moisture accelerate the decomposition of this compound, and aqueous solutions become yellow over time due to oxidative processes. With moisture present, ki forms a. It’s colorless in its pure form and has a distinctive, sharp, salty taste. Reaction with sodium bitartrate produces a white crystalline precipitate that is soluble. Potassium Iodide Solubility In Oil.