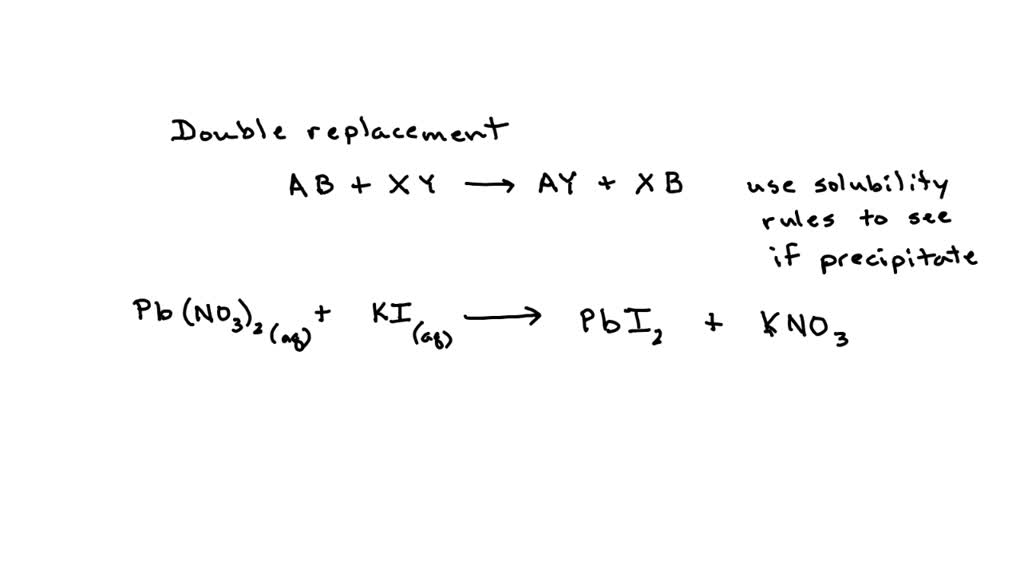
Lead Ii Nitrate And Potassium Iodide Formula . Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. In this text, we use a systematic. For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3).
from www.numerade.com
The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. In this text, we use a systematic. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter.
SOLVED Solutions of lead (II) nitrate and potassium iodide are mixed
Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. In this text, we use a systematic. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a.
From joicffnpl.blob.core.windows.net
Lead Ii Nitrate Reaction With Potassium Iodide at Matilda Lee blog Lead Ii Nitrate And Potassium Iodide Formula Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). For example, the systematic name for kno 3 is potassium nitrate, but. Lead Ii Nitrate And Potassium Iodide Formula.
From joicffnpl.blob.core.windows.net
Lead Ii Nitrate Reaction With Potassium Iodide at Matilda Lee blog Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide. Lead Ii Nitrate And Potassium Iodide Formula.
From www.numerade.com
SOLVED Solutions of lead (II) nitrate and potassium iodide are mixed Lead Ii Nitrate And Potassium Iodide Formula Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals. Lead Ii Nitrate And Potassium Iodide Formula.
From www.numerade.com
SOLVED Reaction of aqueous lead(II) nitrate with aqueous potassium Lead Ii Nitrate And Potassium Iodide Formula For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium. Lead Ii Nitrate And Potassium Iodide Formula.
From www.chegg.com
Solved The precipitation reaction of lead(II) nitrate with Lead Ii Nitrate And Potassium Iodide Formula The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide. Lead Ii Nitrate And Potassium Iodide Formula.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Ii Nitrate And Potassium Iodide Formula Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making. Lead Ii Nitrate And Potassium Iodide Formula.
From www.teachoo.com
MCQ Reema took 5ml of Lead Nitrate solution in a beaker and added ap Lead Ii Nitrate And Potassium Iodide Formula The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Use this demonstration with kit list and safety instructions to. Lead Ii Nitrate And Potassium Iodide Formula.
From www.youtube.com
REACTION OF LEAD NITRATE WITH POTASSIUM IODIDE YouTube Lead Ii Nitrate And Potassium Iodide Formula For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2). Lead Ii Nitrate And Potassium Iodide Formula.
From www.numerade.com
SOLVED If you dissolve lead (II) nitrate and potassium iodide in water Lead Ii Nitrate And Potassium Iodide Formula Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. In this text, we use a systematic. The formula for an ionic compound must contain the same number. Lead Ii Nitrate And Potassium Iodide Formula.
From www.slideserve.com
PPT Unit 2 Basic Chemistry Skills Lesson 9 Percent Yield PowerPoint Lead Ii Nitrate And Potassium Iodide Formula Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes. Lead Ii Nitrate And Potassium Iodide Formula.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Lead Ii Nitrate And Potassium Iodide Formula Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. The reaction, known as the “golden rain” experiment, produces beautiful. Lead Ii Nitrate And Potassium Iodide Formula.
From yazmingokefoster.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide Lead Ii Nitrate And Potassium Iodide Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). For example, the systematic name for kno 3 is potassium nitrate, but its common. Lead Ii Nitrate And Potassium Iodide Formula.
From www.numerade.com
SOLVED 2.Aqueous solutions of aqueous lead(II) nitrate and sodium Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead nitrate reacts. Lead Ii Nitrate And Potassium Iodide Formula.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Lead Ii Nitrate And Potassium Iodide Formula The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as. Lead Ii Nitrate And Potassium Iodide Formula.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Lead Ii Nitrate And Potassium Iodide Formula The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The formula for an ionic compound must contain the same number of positive and negative charges close charge. Lead Ii Nitrate And Potassium Iodide Formula.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Lead Ii Nitrate And Potassium Iodide Formula The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. Use this demonstration with kit list and safety instructions to prove that two solids can react together,. Lead Ii Nitrate And Potassium Iodide Formula.
From www.coursehero.com
[Solved] 2. Solutions of lead(II) nitrate and potassium iodide were Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as. Lead Ii Nitrate And Potassium Iodide Formula.
From www.numerade.com
SOLVED What is the balanced equation of a Lead (II) nitrate and Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Use this demonstration with kit list and. Lead Ii Nitrate And Potassium Iodide Formula.
From www.numerade.com
Convert the following into balanced equations (a) When lead(II Lead Ii Nitrate And Potassium Iodide Formula Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that. Lead Ii Nitrate And Potassium Iodide Formula.
From www.slideserve.com
PPT Types of Chemical Reactions Single and Double Displacement Lead Ii Nitrate And Potassium Iodide Formula Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. In this text, we use a systematic. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. For. Lead Ii Nitrate And Potassium Iodide Formula.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Ii Nitrate And Potassium Iodide Formula Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Lead(ii). Lead Ii Nitrate And Potassium Iodide Formula.
From www.chegg.com
Solved Question 7 Solutions of lead(II) nitrate and Lead Ii Nitrate And Potassium Iodide Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. In this text, we use a systematic. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form. Lead Ii Nitrate And Potassium Iodide Formula.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Ii Nitrate And Potassium Iodide Formula For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. In this text, we use a systematic. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Lead nitrate reacts with potassium iodide to produce a beautiful. Lead Ii Nitrate And Potassium Iodide Formula.
From www.youtube.com
007 Potassium Iodide + Lead (II) Nitrate = Lead (II) Iodide Lead Ii Nitrate And Potassium Iodide Formula The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). For example, the systematic name for kno 3 is potassium nitrate, but. Lead Ii Nitrate And Potassium Iodide Formula.
From www.coursehero.com
[Solved] The reaction between lead (ii) nitrate and potassium iodide is Lead Ii Nitrate And Potassium Iodide Formula The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead nitrate reacts with potassium iodide to produce a beautiful. Lead Ii Nitrate And Potassium Iodide Formula.
From kunduz.com
[ANSWERED] Assume that Potassium Iodide and Lead(II) Nitrate are the Lead Ii Nitrate And Potassium Iodide Formula For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. The. Lead Ii Nitrate And Potassium Iodide Formula.
From www.slideshare.net
Chemistry Lead Ii Nitrate And Potassium Iodide Formula Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. In this text, we use a systematic. The reaction, known as the. Lead Ii Nitrate And Potassium Iodide Formula.
From questions.kunduz.com
10 4 points a When aqueous solutions of Chemistry Lead Ii Nitrate And Potassium Iodide Formula Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as. Lead Ii Nitrate And Potassium Iodide Formula.
From www.coursehero.com
[Solved] The reaction between lead (ii) nitrate and potassium iodide is Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form. Lead Ii Nitrate And Potassium Iodide Formula.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation ID5319064 Lead Ii Nitrate And Potassium Iodide Formula Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will show you. Use this demonstration with kit list and safety instructions to prove that two solids can react together, making lead iodide from lead nitrate and potassium iodide. In this text, we use a systematic. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki). Lead Ii Nitrate And Potassium Iodide Formula.
From slideplayer.com
Precipitation Reactions ppt download Lead Ii Nitrate And Potassium Iodide Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. In this text, we use a systematic. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. Lead(ii) nitrate (pb. Lead Ii Nitrate And Potassium Iodide Formula.
From spmchemistry.blog.onlinetuition.com.my
Chemical Equation SPM Chemistry Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as. Lead Ii Nitrate And Potassium Iodide Formula.
From slideplayer.com
Types of Chemical Reactions ppt download Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. Lead nitrate reacts with potassium iodide to produce a beautiful. Lead Ii Nitrate And Potassium Iodide Formula.
From www.compoundchem.com
Chemical Reactions Lead Iodide & 'Golden Rain' Compound Interest Lead Ii Nitrate And Potassium Iodide Formula For example, the systematic name for kno 3 is potassium nitrate, but its common name is saltpeter. In this text, we use a systematic. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). Lead nitrate reacts with potassium iodide to produce a beautiful precipitate, as we will. Lead Ii Nitrate And Potassium Iodide Formula.
From www.numerade.com
SOLVED What is the equation for this? Solid potassium iodide reacts Lead Ii Nitrate And Potassium Iodide Formula In this text, we use a systematic. Lead(ii) nitrate (pb (no 3) 2) interacts with potassium iodide (ki) to form lead(ii) iodide (pbi 2) and potassium nitrate (kno 3). The reaction, known as the “golden rain” experiment, produces beautiful hexagonal crystals of lead iodide that resemble plates of gold, and makes a great chemistry demonstration. For example, the systematic name. Lead Ii Nitrate And Potassium Iodide Formula.