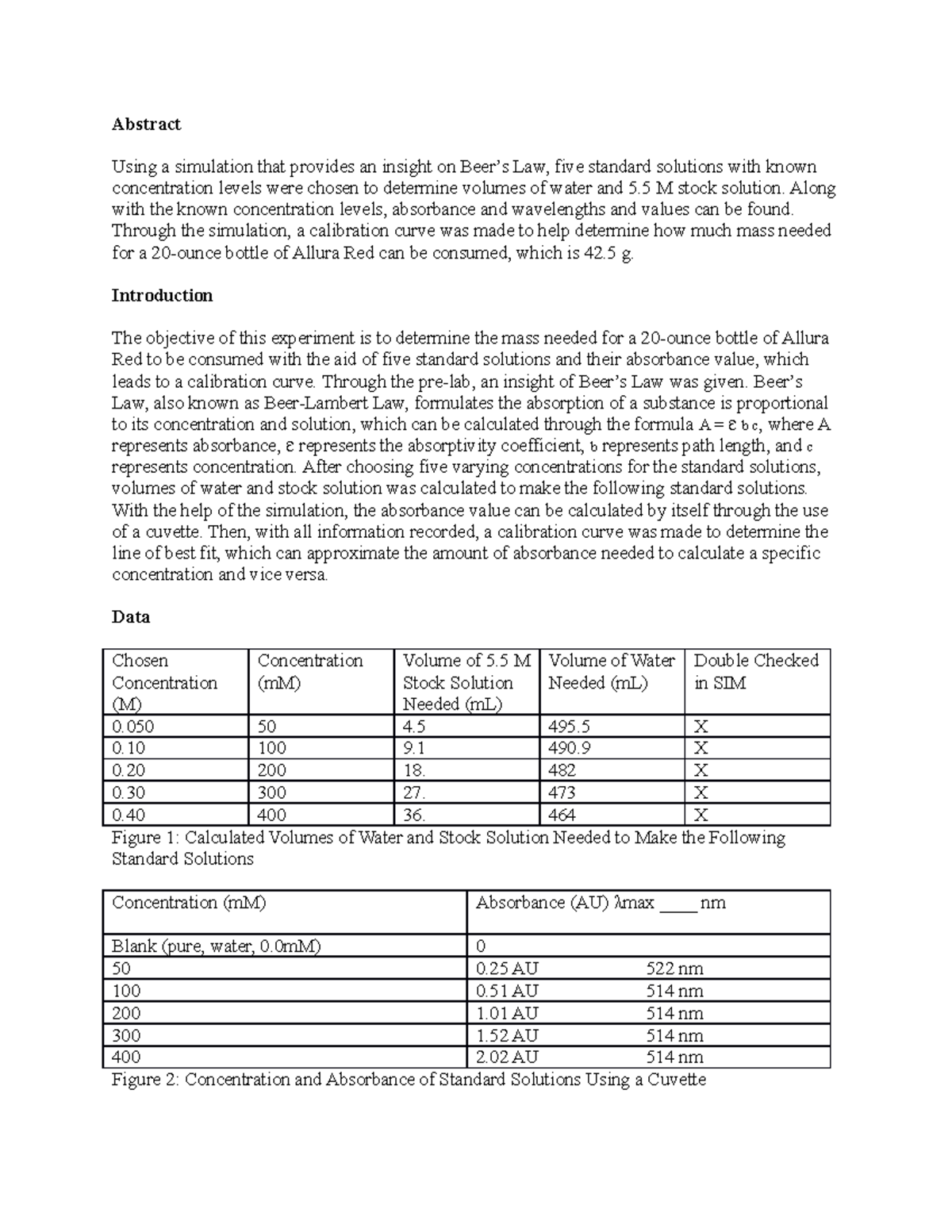
Beer's Law Chem . Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In this section we derive beer's law and consider.
from www.studocu.com
In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. In this section we derive beer's law and consider. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium.
Chem 227 Beer's Law Lap report of beer lab Abstract Using a
Beer's Law Chem Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In this section we derive beer's law and consider.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law Chem Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In this section we derive beer's law and consider. Beer’s law, in spectroscopy,. Beer's Law Chem.
From www.youtube.com
BeerLambert law in easy way YouTube Beer's Law Chem In this section we derive beer's law and consider. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing. Beer's Law Chem.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law connects absorbance to the concentration of the absorbing species. In this section we derive beer's law and consider. Beer’s law, in spectroscopy,. Beer's Law Chem.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer's Law Chem In this section we derive beer's law and consider. Beer's law connects absorbance to the concentration of the absorbing species. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following.. Beer's Law Chem.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint Beer's Law Chem Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. Beer's Law Chem.
From www.youtube.com
Analytical Instrumentation Tutorial 2 Beer Lambert Law YouTube Beer's Law Chem Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In this section we derive beer's law and consider. Beer lamberts law states a relationship between the attenuation of light through a substance and. Beer's Law Chem.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Chem Beer's law connects absorbance to the concentration of the absorbing species. In this section we derive beer's law and consider. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy,. Beer's Law Chem.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the Beer's Law Chem Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law connects absorbance to the concentration of the absorbing species. In this section we derive beer's law and consider. Since the concentration, path. Beer's Law Chem.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law, in spectroscopy,. Beer's Law Chem.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Chem Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity. Beer's Law Chem.
From ecency.com
Spectrophotometry Simplified The BeerLambert Law in Spectrophotom... Beer's Law Chem Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of. Beer's Law Chem.
From www.studocu.com
CHEM 112 Experiment 4 Beer's Law Lab Report Chem 112 queensu Beer's Law Chem Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of. Beer's Law Chem.
From www.studypool.com
SOLUTION Beer lambert law, definition, history, derivation, diagram Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In this section we derive beer's law and consider. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the. Beer's Law Chem.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Chem Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law connects absorbance to the concentration of the absorbing species. Beer’s law, in spectroscopy, a relation concerning the absorption. Beer's Law Chem.
From chemistrysources.com
قانون بيير (بير) Beer’s Law التعريف و المعادلة مصادر الكيمياء Beer's Law Chem Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In this section we derive beer's law and consider. Beer lamberts law states a relationship between the attenuation of light through a substance and. Beer's Law Chem.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Chem Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law connects absorbance. Beer's Law Chem.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a. Beer's Law Chem.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer's Law Chem Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In this section we derive beer's law and consider. Beer lamberts law states a relationship between the. Beer's Law Chem.
From www.studocu.com
Chem 181 4 Beer's Law Tagged Figure 1 Chem 181 Experiment 4 Beer's Law Chem Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In this section we derive beer's law and consider. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Beer's Law Chem.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Chem Beer's law connects absorbance to the concentration of the absorbing species. In this section we derive beer's law and consider. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar. Beer's Law Chem.
From www.studocu.com
Chem 227 Beer's Law Lap report of beer lab Abstract Using a Beer's Law Chem In this section we derive beer's law and consider. Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states. Beer's Law Chem.
From www.youtube.com
Beer's Law Lab Procedure YouTube Beer's Law Chem Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law Chem.
From www.youtube.com
Chem 12 11 Beer's Law YouTube Beer's Law Chem In this section we derive beer's law and consider. Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy,. Beer's Law Chem.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 Beer's Law Chem Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In this section we derive beer's law and consider. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. Beer’s law, in spectroscopy,. Beer's Law Chem.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In this section we derive beer's law and consider. Beer's law connects absorbance to the concentration of the absorbing species. Beer lamberts law states. Beer's Law Chem.
From web.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Beer's Law (BeerLambert Law) Beer's Law Chem Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In this section we derive beer's law and consider. Beer's law connects absorbance to the concentration of the absorbing species. In most experiments, molar. Beer's Law Chem.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the. Beer's Law Chem.
From app.formative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In this section we derive beer's law and consider. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that. Beer's Law Chem.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer's Law Chem Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law connects absorbance to the concentration of the absorbing species. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption. Beer's Law Chem.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Chem Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In this section we derive beer's law and consider. Beer's law connects absorbance to the concentration of the absorbing species. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance.. Beer's Law Chem.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law connects absorbance to the concentration of the absorbing species. In this section we derive beer's law and consider. Beer lamberts law states a relationship between the. Beer's Law Chem.
From www.youtube.com
Chemical Equilibrium & Beer's Law Intro & Theory YouTube Beer's Law Chem In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Beer's law connects absorbance to the concentration of the absorbing species. In this section we derive beer's law and consider. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the. Beer's Law Chem.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer's Law Chem In this section we derive beer's law and consider. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a). Beer's Law Chem.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer's Law Chem Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In this section we. Beer's Law Chem.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Chem Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this section we derive beer's law and consider. Beer lamberts law states a relationship between the attenuation of light through a substance and. Beer's Law Chem.