Water Dilution Formula . Concentration is the removal of solvent, which. Using the dilution equation, we write: the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. You dilute the solution by. V1 is the volume of the starting solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. suppose that you have \(100. \text{ml}\) of a \(2.0 \: state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. C1 is the concentration of the starting solution.
from pressurewashingresource.com
state whether the concentration of a solution is directly or indirectly proportional to its volume. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Concentration is the removal of solvent, which. V1 is the volume of the starting solution. suppose that you have \(100. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. You dilute the solution by. C1 is the concentration of the starting solution. Using the dilution equation, we write:
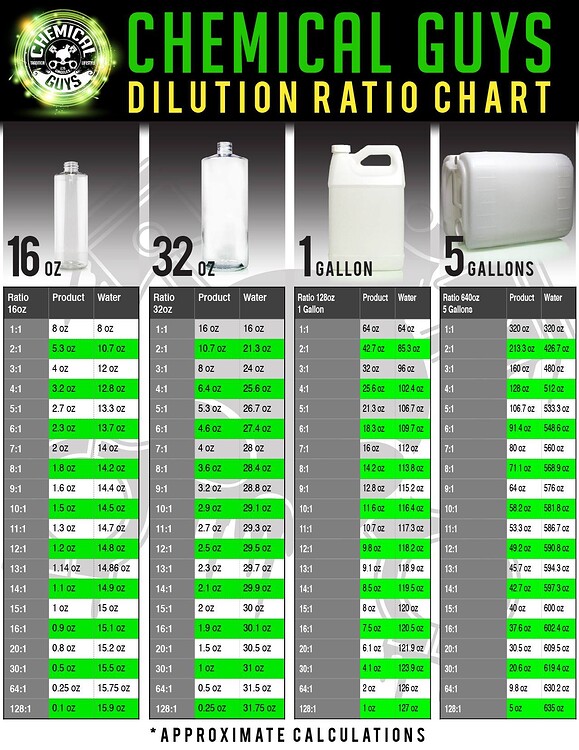
Chem dilution chart 2 by Chemical/Chemistry
Water Dilution Formula V1 is the volume of the starting solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Concentration is the removal of solvent, which. state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Using the dilution equation, we write: C1 is the concentration of the starting solution. suppose that you have \(100. \text{ml}\) of a \(2.0 \: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You dilute the solution by. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. V1 is the volume of the starting solution. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration.
From giobwgqpk.blob.core.windows.net
Dilution Formula Explained at Patti Tejada blog Water Dilution Formula state whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. V1 is the volume of the. Water Dilution Formula.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Water Dilution Formula the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. You dilute the solution by. V1 is the volume of the starting solution. dilution is the addition of solvent, which decreases the concentration of the solute. Water Dilution Formula.
From gioextcnr.blob.core.windows.net
Dilution Chemical Change at Benjamin Marsh blog Water Dilution Formula Using the dilution equation, we write: dilution is the addition of solvent, which decreases the concentration of the solute in the solution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. the formula for calculating a. Water Dilution Formula.
From www.youtube.com
How to Calculate Dilution Factor YouTube Water Dilution Formula the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. C1 is the concentration of the starting solution. V1 is the volume of the starting solution. \text{ml}\) of a \(2.0 \: Using the dilution equation, we write:. Water Dilution Formula.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Water Dilution Formula the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Using the dilution equation, we write: C1 is the concentration of the starting solution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x. Water Dilution Formula.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Water Dilution Formula the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. suppose that you have \(100. Concentration is the removal of solvent, which. \text{ml}\) of a \(2.0 \: V1 is the volume of the starting solution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. C1 is the. Water Dilution Formula.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Water Dilution Formula \text{ml}\) of a \(2.0 \: V1 is the volume of the starting solution. You dilute the solution by. Concentration is the removal of solvent, which. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. state whether the concentration of a solution is directly or indirectly proportional to its volume.. Water Dilution Formula.
From pressurewashingresource.com
Chem dilution chart 2 by Chemical/Chemistry Water Dilution Formula You dilute the solution by. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Using the dilution equation, we write: Concentration. Water Dilution Formula.
From www.youtube.com
Dilution Method Problems Waste Water Engineering YouTube Water Dilution Formula dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. You dilute the solution by. C1 is the concentration of the starting solution. suppose that you have \(100. Concentration is the removal of solvent, which. \text{ml}\) of a. Water Dilution Formula.
From www.slideshare.net
Molarity and dilution Water Dilution Formula V1 is the volume of the starting solution. Concentration is the removal of solvent, which. You dilute the solution by. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. \text{ml}\) of a \(2.0 \: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. suppose that you have. Water Dilution Formula.
From chem2u.blogspot.com
chem2U Dilution method formula Water Dilution Formula Concentration is the removal of solvent, which. You dilute the solution by. state whether the concentration of a solution is directly or indirectly proportional to its volume. Using the dilution equation, we write: the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. dilution is the addition of solvent, which decreases the concentration of. Water Dilution Formula.
From exyzklqnz.blob.core.windows.net
Dilution Calculation Equations at Rebecca Mathis blog Water Dilution Formula the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. \text{ml}\) of a \(2.0 \: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Using the dilution equation, we write: suppose that you have \(100. V1. Water Dilution Formula.
From giowqjocn.blob.core.windows.net
Dilution Formula Define at John Vaughn blog Water Dilution Formula Using the dilution equation, we write: the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. V1 is the volume of the starting solution. You dilute the solution by. \text{ml}\) of a \(2.0 \: the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. C1 is. Water Dilution Formula.
From www.slideserve.com
PPT Molarity, Dilution, and pH PowerPoint Presentation, free download Water Dilution Formula C1 is the concentration of the starting solution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. suppose that you have \(100. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Concentration. Water Dilution Formula.
From www.slideshare.net
Molarity and dilution Water Dilution Formula Concentration is the removal of solvent, which. state whether the concentration of a solution is directly or indirectly proportional to its volume. C1 is the concentration of the starting solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. \text{ml}\) of a \(2.0 \: You dilute the solution by. the. Water Dilution Formula.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Water Dilution Formula Concentration is the removal of solvent, which. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. V1 is the volume of the starting solution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. suppose that you have \(100. the formula for calculating a dilution is (c1). Water Dilution Formula.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Water Dilution Formula the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. You dilute the solution by. Concentration is the removal of solvent, which. \text{ml}\) of a \(2.0 \: suppose that you. Water Dilution Formula.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Water Dilution Formula Concentration is the removal of solvent, which. C1 is the concentration of the starting solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. suppose that you have \(100. Using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. You dilute the solution by. \text{ml}\). Water Dilution Formula.
From www.slideserve.com
PPT Water The Universal Solvent PowerPoint Presentation, free Water Dilution Formula (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. Using the dilution equation, we write: You dilute the solution by. V1 is the volume of the starting solution. Concentration is the removal of solvent, which. \text{ml}\) of a \(2.0. Water Dilution Formula.
From www.slideserve.com
PPT Making Molar Solutions PowerPoint Presentation, free download Water Dilution Formula the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. V1 is the volume of the starting solution. You dilute the solution by. \text{ml}\) of a \(2.0 \: dilution is. Water Dilution Formula.
From www.youtube.com
How to Perform a Bacterial Dilution Calculation YouTube Water Dilution Formula suppose that you have \(100. You dilute the solution by. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. state whether the concentration of a solution is directly or indirectly proportional. Water Dilution Formula.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free Water Dilution Formula dilution is the addition of solvent, which decreases the concentration of the solute in the solution. V1 is the volume of the starting solution. You dilute the solution by. suppose that you have \(100. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. state whether the concentration of a solution is directly. Water Dilution Formula.
From exyzklqnz.blob.core.windows.net
Dilution Calculation Equations at Rebecca Mathis blog Water Dilution Formula dilution is the addition of solvent, which decreases the concentration of the solute in the solution. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. V1 is the volume. Water Dilution Formula.
From giowrfocz.blob.core.windows.net
Dilution Instructions at Dawn Morey blog Water Dilution Formula the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. \text{ml}\) of a \(2.0 \: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. You dilute the solution by. C1 is the concentration of the starting solution. the dilution ratio calculator tells you how much solute and solvent. Water Dilution Formula.
From www.slideserve.com
PPT Dilution PowerPoint Presentation, free download ID6016027 Water Dilution Formula (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Concentration is the removal of solvent, which. C1 is the concentration of the starting solution. \text{ml}\) of a \(2.0 \: the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. suppose that you have \(100. dilution is the. Water Dilution Formula.
From printablelibbulges.z21.web.core.windows.net
How To Calculate Dilution Concentrations Water Dilution Formula C1 is the concentration of the starting solution. Using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. You dilute the solution by. the formula for calculating a dilution is (c1) (v1) =. Water Dilution Formula.
From www.youtube.com
Molarity and Dilution Calculations YouTube Water Dilution Formula state whether the concentration of a solution is directly or indirectly proportional to its volume. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Concentration is the removal of solvent, which. V1 is the volume of the starting solution. the water dilution calculator helps determine the amount of water needed to dilute a. Water Dilution Formula.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Water Dilution Formula V1 is the volume of the starting solution. \text{ml}\) of a \(2.0 \: C1 is the concentration of the starting solution. You dilute the solution by. state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution ratio calculator tells you how much solute and solvent you need to get the desired. Water Dilution Formula.
From www.researchgate.net
Exactly why do we dilute waste water samples to find BOD? and how to Water Dilution Formula Using the dilution equation, we write: C1 is the concentration of the starting solution. Concentration is the removal of solvent, which. state whether the concentration of a solution is directly or indirectly proportional to its volume. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. suppose that you have \(100. the dilution ratio calculator tells. Water Dilution Formula.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Water Dilution Formula the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. V1 is the volume of the starting solution. \text{ml}\) of a \(2.0 \: Using the dilution equation, we write: Concentration is the removal of solvent, which. You dilute the solution by. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x. Water Dilution Formula.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Water Dilution Formula Using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. You dilute the solution by. C1 is the concentration of the starting solution. the water dilution calculator helps determine the amount of water needed to dilute a solution from one concentration. the formula for calculating a dilution is (c1) (v1) =. Water Dilution Formula.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Water Dilution Formula the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Using the dilution equation, we write: the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. (1.50 mol/l) (53.4. Water Dilution Formula.
From www.slideserve.com
PPT Concentration of Solutions PowerPoint Presentation, free download Water Dilution Formula C1 is the concentration of the starting solution. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. suppose that you have \(100. the dilution ratio calculator tells you how much solute and solvent you need to get the desired. Water Dilution Formula.
From ar.inspiredpencil.com
Dilution Equation Water Dilution Formula Concentration is the removal of solvent, which. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. state whether the concentration of a solution is directly or indirectly proportional to its volume. Using the dilution equation, we write: C1 is the concentration of the starting solution. V1 is the volume of the starting solution. dilution is the. Water Dilution Formula.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Water Dilution Formula Concentration is the removal of solvent, which. C1 is the concentration of the starting solution. \text{ml}\) of a \(2.0 \: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. suppose that you have \(100. Using the dilution equation, we write: dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Water Dilution Formula.