How Does Concentration Of Buffer Affect Buffer Capacity . Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. How do buffer solutions work? How to calculate buffer capacity? The goal of a buffer is to keep the ph of a solution within a narrow range. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. Before we get into what a buffer capacity is, we should first understand buffers. In addition to the pk a value,. What factors determine the effectiveness (or capacity) of a buffer? The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit.
from www.slideserve.com
Before we get into what a buffer capacity is, we should first understand buffers. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. What factors determine the effectiveness (or capacity) of a buffer? In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. How to calculate buffer capacity? The goal of a buffer is to keep the ph of a solution within a narrow range. Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. How do buffer solutions work? In addition to the pk a value,.
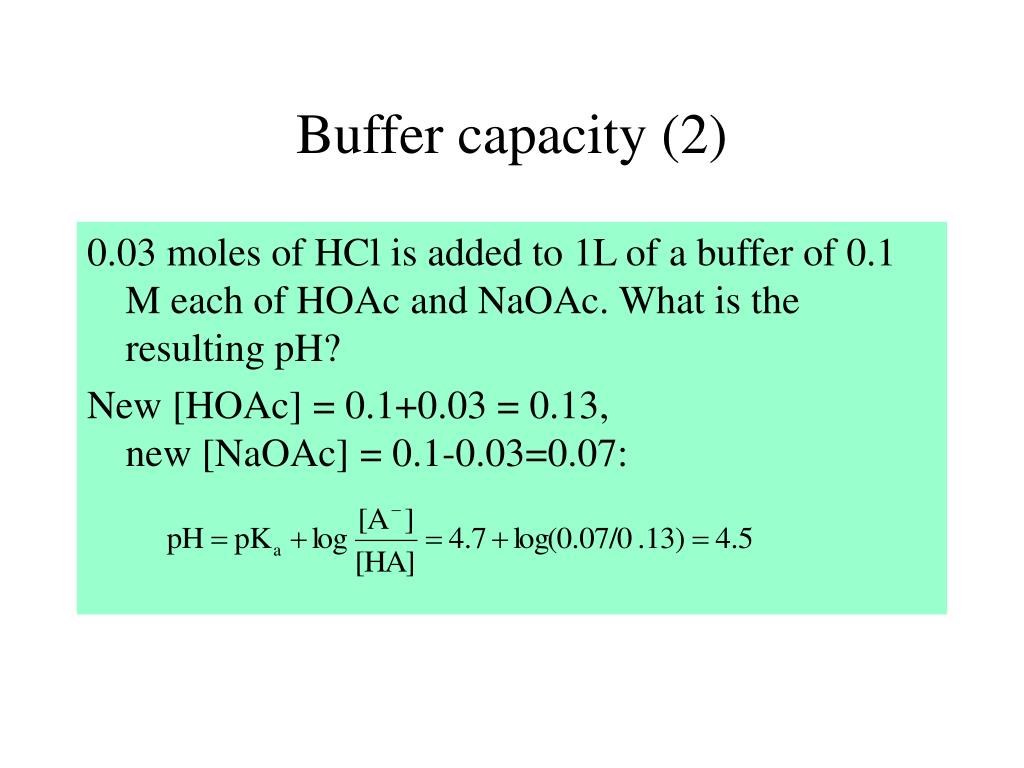
PPT Chem 1310 Introduction to physical chemistry Part 5 Buffers and
How Does Concentration Of Buffer Affect Buffer Capacity In addition to the pk a value,. The goal of a buffer is to keep the ph of a solution within a narrow range. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. Before we get into what a buffer capacity is, we should first understand buffers. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. In addition to the pk a value,. Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. What factors determine the effectiveness (or capacity) of a buffer? Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. How to calculate buffer capacity? How do buffer solutions work?
From www.slideserve.com
PPT Chem 1310 Introduction to physical chemistry Part 5 Buffers and How Does Concentration Of Buffer Affect Buffer Capacity Before we get into what a buffer capacity is, we should first understand buffers. Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. How to calculate buffer capacity? What factors determine the effectiveness (or capacity) of a buffer? The buffer capacity is the amount of acid. How Does Concentration Of Buffer Affect Buffer Capacity.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts How Does Concentration Of Buffer Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that. How Does Concentration Of Buffer Affect Buffer Capacity.
From en.ppt-online.org
Buffer solutions online presentation How Does Concentration Of Buffer Affect Buffer Capacity Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. The goal of a buffer is. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free How Does Concentration Of Buffer Affect Buffer Capacity The goal of a buffer is to keep the ph of a solution within a narrow range. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. What factors determine the effectiveness. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideshare.net
02 hydrolysis. buffers__colloids How Does Concentration Of Buffer Affect Buffer Capacity Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. In addition to the pk a value,. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID How Does Concentration Of Buffer Affect Buffer Capacity Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. In addition to the pk a value,. The goal of a buffer is to keep the ph of a solution within a narrow range. What factors determine the effectiveness (or capacity) of. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Buffer Capacity Complexation PowerPoint Presentation, free How Does Concentration Of Buffer Affect Buffer Capacity How to calculate buffer capacity? Before we get into what a buffer capacity is, we should first understand buffers. Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. How do buffer solutions work? In more rigorous terms, buffer capacity is defined. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID720100 How Does Concentration Of Buffer Affect Buffer Capacity Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. How do buffer solutions work? In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint How Does Concentration Of Buffer Affect Buffer Capacity Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. What. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent How Does Concentration Of Buffer Affect Buffer Capacity How to calculate buffer capacity? Before we get into what a buffer capacity is, we should first understand buffers. In addition to the pk a value,. The goal of a buffer is to keep the ph of a solution within a narrow range. Typically, the buffer of choice should have a pk a value that is within plus or minus. How Does Concentration Of Buffer Affect Buffer Capacity.
From users.highland.edu
Buffers How Does Concentration Of Buffer Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. How do buffer solutions work? In addition to the pk a value,. Although the useful ph range of a buffer depends strongly on the chemical properties of the weak. How Does Concentration Of Buffer Affect Buffer Capacity.
From study.com
Identifying Buffer Capacity Chemistry How Does Concentration Of Buffer Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? How to calculate buffer capacity? In addition to the pk a value,. Before we get into what a buffer capacity is, we should first understand buffers. Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.numerade.com
SOLVED The Buffer Capacity The capacity of a buffer depends not only How Does Concentration Of Buffer Affect Buffer Capacity In addition to the pk a value,. How do buffer solutions work? The goal of a buffer is to keep the ph of a solution within a narrow range. Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. In more rigorous terms, buffer capacity is defined. How Does Concentration Of Buffer Affect Buffer Capacity.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online How Does Concentration Of Buffer Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. In. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer How Does Concentration Of Buffer Affect Buffer Capacity Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. Before we get into what a buffer capacity is, we should first understand buffers. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added. How Does Concentration Of Buffer Affect Buffer Capacity.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG How Does Concentration Of Buffer Affect Buffer Capacity Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. The goal of a buffer is to keep the ph of a solution within a narrow range. In addition to the pk a value,. The buffer capacity is the amount of acid. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.chegg.com
Solved 1. How does the concentration of the buffer affect How Does Concentration Of Buffer Affect Buffer Capacity The goal of a buffer is to keep the ph of a solution within a narrow range. Before we get into what a buffer capacity is, we should first understand buffers. In addition to the pk a value,. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.youtube.com
Buffer and buffer capacity Revised Biochemistry YouTube How Does Concentration Of Buffer Affect Buffer Capacity Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. In addition to the pk a value,. Before we get into what a buffer capacity is, we should first understand buffers. The goal of a buffer is to keep the ph of. How Does Concentration Of Buffer Affect Buffer Capacity.
From facts.net
13 Enigmatic Facts About Buffer Capacity How Does Concentration Of Buffer Affect Buffer Capacity Before we get into what a buffer capacity is, we should first understand buffers. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Typically, the buffer of choice should have a. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation, free download How Does Concentration Of Buffer Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? How to calculate buffer capacity? Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.youtube.com
1503 buffers part 2 selection and combined concentration YouTube How Does Concentration Of Buffer Affect Buffer Capacity How to calculate buffer capacity? Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. How. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free How Does Concentration Of Buffer Affect Buffer Capacity Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its. How Does Concentration Of Buffer Affect Buffer Capacity.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online How Does Concentration Of Buffer Affect Buffer Capacity Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. The goal of a buffer is to keep the ph of a solution within a narrow range. What factors determine the effectiveness (or capacity) of a buffer? In more rigorous terms, buffer capacity is defined as the. How Does Concentration Of Buffer Affect Buffer Capacity.
From chemexpert.net
Mastering Buffer Capacity Your Guide To Maintaining Chemical Harmony How Does Concentration Of Buffer Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. How do buffer solutions work? In addition to the pk a value,. Typically, the buffer of choice should have a pk a value that is within plus or minus. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Chapter Two Water The Solvent for Biochemical Reactions How Does Concentration Of Buffer Affect Buffer Capacity How do buffer solutions work? Before we get into what a buffer capacity is, we should first understand buffers. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. The buffer capacity. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 How Does Concentration Of Buffer Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. How. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.youtube.com
Buffer range and capacity YouTube How Does Concentration Of Buffer Affect Buffer Capacity The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. In addition to the pk a value,. Before we get into what a buffer capacity is, we should first understand buffers. In more rigorous terms, buffer capacity is defined. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free How Does Concentration Of Buffer Affect Buffer Capacity What factors determine the effectiveness (or capacity) of a buffer? In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Although the useful ph range of a buffer depends strongly on the. How Does Concentration Of Buffer Affect Buffer Capacity.
From hillalifeller.blogspot.com
What Makes A Good Buffer System Hill Alifeller How Does Concentration Of Buffer Affect Buffer Capacity How do buffer solutions work? In addition to the pk a value,. The goal of a buffer is to keep the ph of a solution within a narrow range. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free How Does Concentration Of Buffer Affect Buffer Capacity In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Before we get into what a buffer capacity is, we should first understand buffers. Although the useful ph range of a buffer. How Does Concentration Of Buffer Affect Buffer Capacity.
From pdfprof.com
buffer capacity experiment procedure How Does Concentration Of Buffer Affect Buffer Capacity How to calculate buffer capacity? The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. In. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.studypool.com
SOLUTION 4 buffering capacity Studypool How Does Concentration Of Buffer Affect Buffer Capacity Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. How do buffer solutions work? The goal of a buffer is to keep the ph of a solution within a narrow range. The buffer capacity is the amount of acid or base. How Does Concentration Of Buffer Affect Buffer Capacity.
From relationshipbetween.com
Difference Between Buffer Action And Buffer Capacity Relationship Between How Does Concentration Of Buffer Affect Buffer Capacity Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and weak base used to prepare the buffer (i.e., on. Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. How do buffer solutions work? The goal of a. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.youtube.com
Buffer capacity; making a buffer with a given pH value YouTube How Does Concentration Of Buffer Affect Buffer Capacity Typically, the buffer of choice should have a pk a value that is within plus or minus 1 unit of the desired ph. In addition to the pk a value,. How to calculate buffer capacity? The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph. How Does Concentration Of Buffer Affect Buffer Capacity.
From www.slideserve.com
PPT Chapter 13 Applications of Aqueous Equilibria PowerPoint How Does Concentration Of Buffer Affect Buffer Capacity How to calculate buffer capacity? The buffer capacity is the amount of acid or base that can be added to a given volume of a buffer solution before the ph changes significantly, usually by one unit. How do buffer solutions work? Although the useful ph range of a buffer depends strongly on the chemical properties of the weak acid and. How Does Concentration Of Buffer Affect Buffer Capacity.