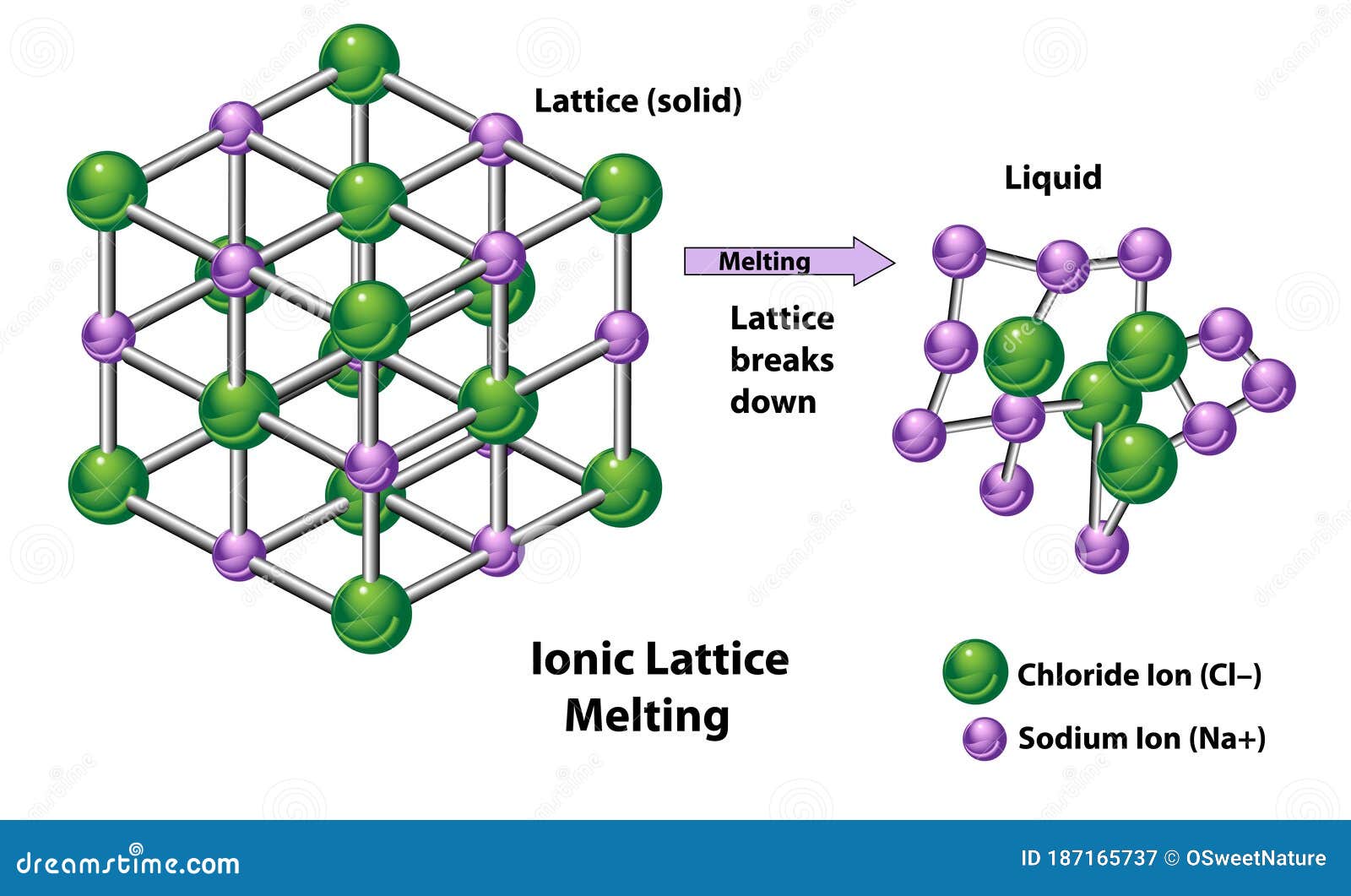
Ionic Crystal Melting Point . The process of melting an ionic. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. See the study guide on the three. Ionic crystals are hard and brittle and have high melting points. Melting point and boiling point:
from www.dreamstime.com
Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Ionic crystals are hard and brittle and have high melting points. Melting point and boiling point: The process of melting an ionic. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. See the study guide on the three.
Ionic Lattice Process of Melting Stock Vector Illustration of binds
Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. The process of melting an ionic. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. See the study guide on the three. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. Ionic crystals are hard and brittle and have high melting points. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Melting point and boiling point:
From getchemistry.weebly.com
Ionic Bonding Chemistry Ionic Crystal Melting Point Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. The process of melting an ionic. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. Ionic solids, such as sodium chloride and nickel oxide, are composed. Ionic Crystal Melting Point.
From www.youtube.com
Melting point of ionic and molecular solids. YouTube Ionic Crystal Melting Point Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. Melting point and boiling point: See the study guide on the three.. Ionic Crystal Melting Point.
From www.youtube.com
GCSE Chemistry 19 Why do Ionic Compounds have High Melting Points Ionic Crystal Melting Point Ionic crystals are hard and brittle and have high melting points. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. The process of melting an ionic. See the. Ionic Crystal Melting Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Ionic Crystal Melting Point Melting point and boiling point: Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. The process of melting an ionic. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic crystals are hard and brittle and have high melting points. See the. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Properties of Ionic Bonds PowerPoint Presentation, free download Ionic Crystal Melting Point Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Ionic crystals are hard and brittle and have high melting points. See the study guide on the three. The process of melting an ionic. Because of the many simultaneous attractions between cations and anions that occur, ionic. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Heating Curve PowerPoint Presentation, free download ID5007002 Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Generally,. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID2090175 Ionic Crystal Melting Point The process of melting an ionic. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. See the study guide on the three. Ionic crystals are hard and brittle and have high melting points. Melting point and boiling point: Since the ions are strongly bounded to each other by strong electrostatic force. Ionic Crystal Melting Point.
From www.youtube.com
Compare Melting Point of Ionic Compounds YouTube Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Melting point and boiling point: Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Since the ions are strongly bounded to each other by strong electrostatic force of. Ionic Crystal Melting Point.
From slideplayer.com
Ionic Bonds Chapter 5 Section ppt download Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. Melting point and boiling point: Because of the many simultaneous attractions between cations and anions that occur, ionic crystal. Ionic Crystal Melting Point.
From slideplayer.com
Chemical Bonding. ppt download Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. See the study guide on the three. Melting point and boiling point: Ionic compounds have high melting. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint Ionic Crystal Melting Point See the study guide on the three. Melting point and boiling point: Ionic crystals are hard and brittle and have high melting points. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals. Ionic Crystal Melting Point.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic. Ionic Crystal Melting Point.
From chem.libretexts.org
Chapter 4.2 Lattice Energies in Ionic Solids Chemistry LibreTexts Ionic Crystal Melting Point Ionic crystals are hard and brittle and have high melting points. The process of melting an ionic. Melting point and boiling point: Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. See the study guide on the three. Because of the many simultaneous attractions between cations. Ionic Crystal Melting Point.
From slidetodoc.com
Section 2 Ionic Bonds and Ionic Compounds Oppositely Ionic Crystal Melting Point Ionic crystals are hard and brittle and have high melting points. See the study guide on the three. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Melting point and boiling point: The process of melting an ionic. Because of the many simultaneous attractions between cations. Ionic Crystal Melting Point.
From sciencenotes.org
Melting Point Definition and List Ionic Crystal Melting Point Ionic crystals are hard and brittle and have high melting points. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. See the study guide on the. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Solids differ Hardness Melting point Flexibility Conductivity Ionic Crystal Melting Point Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. See the study guide on the three. Ionic solids, such as sodium chloride and nickel oxide, are composed of. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint Ionic Crystal Melting Point Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal. Ionic Crystal Melting Point.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Ionic Crystal Melting Point Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. The process of melting an ionic. See the study guide on the three. Melting point and boiling point: Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very. Ionic Crystal Melting Point.
From www.nuclear-power.com
Melting Point of Materials Ionic Crystal Melting Point The process of melting an ionic. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. See the study guide on the three. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic compounds have high. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Solids PowerPoint Presentation, free download ID9472667 Ionic Crystal Melting Point See the study guide on the three. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very. Ionic Crystal Melting Point.
From mammothmemory.net
Ionic compounds have a very high melting point and dissolve Ionic Crystal Melting Point Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Ionic crystals are hard and brittle and have high melting points. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic compounds have high melting and boiling points,. Ionic Crystal Melting Point.
From www.dreamstime.com
Ionic Lattice Process of Melting Stock Vector Illustration of binds Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. The process of melting an ionic. Melting point and boiling point: Generally, ionic crystals form from a combination of group 1 or 2 metals. Ionic Crystal Melting Point.
From www.teachoo.com
Why do ionic compounds have high melting points? Teachoo Science Ionic Crystal Melting Point Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. Ionic solids, such as sodium chloride and nickel oxide, are composed of. Ionic Crystal Melting Point.
From wagine.com
17 Metals With the Highest Melting Points (and Why) Materials Science Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. Melting point and boiling point: Ionic crystals are hard and brittle and have high melting points. See the study. Ionic Crystal Melting Point.
From slideplayer.com
Ionic Solids 201 Chemistry. ppt download Ionic Crystal Melting Point Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. See the study guide on the three. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Because of the many simultaneous attractions between cations and anions that occur,. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Properties of Solids PowerPoint Presentation, free download ID Ionic Crystal Melting Point The process of melting an ionic. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Ionic crystals are hard and brittle and have high melting points. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or. Ionic Crystal Melting Point.
From www.researchgate.net
4 Melting points of some ionic liquids and salts. Download Ionic Crystal Melting Point Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or nonmetallic polyatomic ions. The process of melting an ionic. Since the ions are strongly bounded to each. Ionic Crystal Melting Point.
From www.ck12.org
Comparing Crystals Overview ( Video ) Chemistry CK12 Foundation Ionic Crystal Melting Point See the study guide on the three. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic crystals are hard and brittle and have high melting points. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Because. Ionic Crystal Melting Point.
From courses.lumenlearning.com
Ionic Crystals Introduction to Chemistry Ionic Crystal Melting Point The process of melting an ionic. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. Ionic crystals are hard and brittle and have high melting points.. Ionic Crystal Melting Point.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download Ionic Crystal Melting Point Ionic crystals are hard and brittle and have high melting points. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. See the study guide on the. Ionic Crystal Melting Point.
From www.youtube.com
Melting Point Trends of Ionic Compounds YouTube Ionic Crystal Melting Point Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Ionic crystals are hard and brittle and have high melting points. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Ionic compounds have high melting and boiling points,. Ionic Crystal Melting Point.
From chem.libretexts.org
Chapter 4.2 Lattice Energies in Ionic Solids Chemistry LibreTexts Ionic Crystal Melting Point Ionic crystals are hard and brittle and have high melting points. See the study guide on the three. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals. Ionic Crystal Melting Point.
From slideplayer.com
Solids Chem ppt download Ionic Crystal Melting Point Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this. Ionic Crystal Melting Point.
From www.chemistrystudent.com
Lattice Enthalpies (Alevel) ChemistryStudent Ionic Crystal Melting Point The process of melting an ionic. Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions,. Ionic crystals are hard and brittle and have high melting points. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Generally, ionic. Ionic Crystal Melting Point.
From slideplayer.com
Explaining the Physical Properties of Ionic Substances ppt download Ionic Crystal Melting Point Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. Since the ions are strongly bounded to each other by strong electrostatic force of attraction, large amount of energy is required to overcom this attraction. The process of melting an ionic. Generally, ionic crystals form from a combination of group 1 or. Ionic Crystal Melting Point.