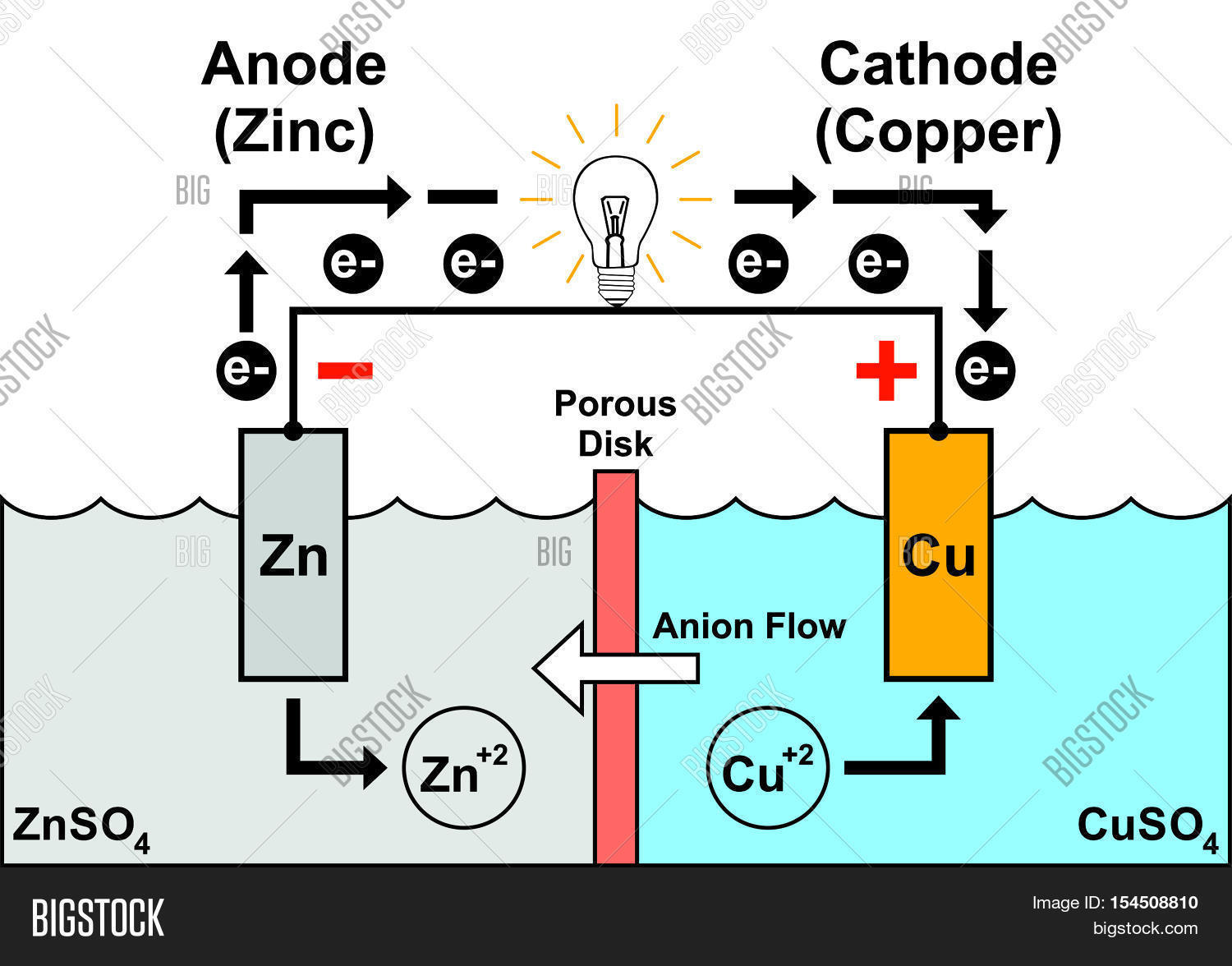
Positive Electrode In Galvanic Cell . Indicate which electrode is the positive. This seems reasonable as the anode is the source of. Electrons can flow through an external wire and become available to do electrical work. Electrons can flow through an external wire and become available to do electrical work. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Indicate which electrode is the cathode and which is the anode. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left.
from www.bigstockphoto.com
In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Electrons can flow through an external wire and become available to do electrical work. This seems reasonable as the anode is the source of. Indicate which electrode is the cathode and which is the anode. Electrons can flow through an external wire and become available to do electrical work. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Indicate which electrode is the positive.
Galvanic Cell Simple & Easy Image & Photo Bigstock
Positive Electrode In Galvanic Cell With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Electrons can flow through an external wire and become available to do electrical work. Indicate which electrode is the cathode and which is the anode. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. This seems reasonable as the anode is the source of. Electrons can flow through an external wire and become available to do electrical work. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Indicate which electrode is the positive.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of Positive Electrode In Galvanic Cell This seems reasonable as the anode is the source of. Electrons can flow through an external wire and become available to do electrical work. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Indicate which electrode is the positive. Indicate which electrode is. Positive Electrode In Galvanic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Positive Electrode In Galvanic Cell This seems reasonable as the anode is the source of. Indicate which electrode is the positive. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Electrons can flow through an external wire and become available to do electrical work. With this mnemonic you. Positive Electrode In Galvanic Cell.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary Positive Electrode In Galvanic Cell With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Electrons can flow through an external wire and become available to do electrical work. This seems reasonable as the anode is the source of. In a. Positive Electrode In Galvanic Cell.
From www.coursehero.com
[Solved] draw a galvanic cell and label the anode/cathode, cell Positive Electrode In Galvanic Cell This seems reasonable as the anode is the source of. Indicate which electrode is the cathode and which is the anode. Indicate which electrode is the positive. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Electrons can flow through an external wire and become available to do electrical work. Electrons can flow. Positive Electrode In Galvanic Cell.
From biochemreview.weebly.com
Galvanic cell BIOChemReview Positive Electrode In Galvanic Cell When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Indicate which electrode is the positive. Indicate which electrode is the cathode and which is the anode. Electrons can flow through an external wire and become available to do electrical work. This seems reasonable. Positive Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Positive Electrode In Galvanic Cell In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Electrons can flow through an external wire and become available to do electrical work. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Electrons can flow through. Positive Electrode In Galvanic Cell.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Positive Electrode In Galvanic Cell Indicate which electrode is the positive. Indicate which electrode is the cathode and which is the anode. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Electrons can flow through an external wire and become available to do electrical work. This seems reasonable. Positive Electrode In Galvanic Cell.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Positive Electrode In Galvanic Cell Indicate which electrode is the cathode and which is the anode. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Indicate which electrode is the positive. In a galvanic (voltaic) cell, the anode is considered. Positive Electrode In Galvanic Cell.
From ck12.org
In the electrochemical cell illustrated above, the copper metal must be Positive Electrode In Galvanic Cell Indicate which electrode is the positive. Electrons can flow through an external wire and become available to do electrical work. Electrons can flow through an external wire and become available to do electrical work. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. With this mnemonic you can quickly remember for a galvanic. Positive Electrode In Galvanic Cell.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Positive Electrode In Galvanic Cell Electrons can flow through an external wire and become available to do electrical work. Electrons can flow through an external wire and become available to do electrical work. This seems reasonable as the anode is the source of. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. With this mnemonic you can quickly. Positive Electrode In Galvanic Cell.
From www.pinterest.com
Galvanic Cell or Voltaic cell Construction and working Positive Electrode In Galvanic Cell Electrons can flow through an external wire and become available to do electrical work. Indicate which electrode is the positive. Electrons can flow through an external wire and become available to do electrical work. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and. Positive Electrode In Galvanic Cell.
From kladwjlgf.blob.core.windows.net
Electrochemistry Galvanic Cells at Roberto Alvarado blog Positive Electrode In Galvanic Cell When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Indicate which electrode is the positive. Indicate which electrode is the cathode and which is the anode. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Electrons. Positive Electrode In Galvanic Cell.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram Positive Electrode In Galvanic Cell Electrons can flow through an external wire and become available to do electrical work. This seems reasonable as the anode is the source of. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. With this mnemonic you can quickly remember for a galvanic. Positive Electrode In Galvanic Cell.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle Positive Electrode In Galvanic Cell Electrons can flow through an external wire and become available to do electrical work. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Electrons can flow through an external wire and become available to do. Positive Electrode In Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Positive Electrode In Galvanic Cell When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. This seems reasonable as the anode is the source of. Indicate which electrode is the cathode and which is the anode. Electrons can flow through an external wire and become available to do electrical. Positive Electrode In Galvanic Cell.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Positive Electrode In Galvanic Cell With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Indicate which electrode is the cathode and which is the anode. Indicate which electrode is the positive. This seems reasonable as the anode is the source. Positive Electrode In Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Positive Electrode In Galvanic Cell This seems reasonable as the anode is the source of. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Indicate which electrode is the positive. Electrons can flow through an external wire and become available to do electrical work. Indicate which electrode is the cathode and which is the anode. With this mnemonic. Positive Electrode In Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Positive Electrode In Galvanic Cell This seems reasonable as the anode is the source of. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Indicate which electrode is the cathode and which is the anode. With this mnemonic you can quickly remember for a galvanic cell diagram or. Positive Electrode In Galvanic Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Positive Electrode In Galvanic Cell With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Indicate which electrode is the positive. Electrons can flow through an external wire and become available to do electrical work. In a galvanic (voltaic) cell, the. Positive Electrode In Galvanic Cell.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Positive Electrode In Galvanic Cell When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the.. Positive Electrode In Galvanic Cell.
From pediaa.com
Difference Between Daniell Cell and Galvanic Cell Definition, How Positive Electrode In Galvanic Cell Indicate which electrode is the positive. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Electrons can flow through an external wire and become available to do electrical work. Electrons can flow through an external wire and become available to do electrical work. When the electrochemical cell is constructed in this fashion, a. Positive Electrode In Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Positive Electrode In Galvanic Cell When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Indicate which electrode is the cathode and which is the anode. Electrons can flow through an external wire and become available to do electrical work. Indicate which electrode is the positive. With this mnemonic. Positive Electrode In Galvanic Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Positive Electrode In Galvanic Cell When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. Indicate which electrode is the positive. This seems reasonable as the anode is the source of. Electrons can flow through an external wire and become available to do electrical work. Electrons can flow through. Positive Electrode In Galvanic Cell.
From www.slideserve.com
PPT Galvanic and Electrolytic Cells PowerPoint Presentation, free Positive Electrode In Galvanic Cell In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Electrons can flow through an external wire and become available to do electrical work. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the. Positive Electrode In Galvanic Cell.
From www.bigstockphoto.com
Galvanic Cell Simple & Easy Image & Photo Bigstock Positive Electrode In Galvanic Cell In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is. Positive Electrode In Galvanic Cell.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation ID2216847 Positive Electrode In Galvanic Cell In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Electrons can flow through an external wire and become available to do electrical work. This seems reasonable as the anode is the source of. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the. Positive Electrode In Galvanic Cell.
From www.dynamicscience.com.au
chemistry galvanic cells Positive Electrode In Galvanic Cell Electrons can flow through an external wire and become available to do electrical work. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. This seems reasonable as. Positive Electrode In Galvanic Cell.
From dxotvmkyy.blob.core.windows.net
Galvanic Current Positive Negative at Anita Reilly blog Positive Electrode In Galvanic Cell Indicate which electrode is the positive. Electrons can flow through an external wire and become available to do electrical work. This seems reasonable as the anode is the source of. Electrons can flow through an external wire and become available to do electrical work. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous. Positive Electrode In Galvanic Cell.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Positive Electrode In Galvanic Cell In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. This seems reasonable as the anode is the source of. Electrons can flow through an external wire and. Positive Electrode In Galvanic Cell.
From www.pinterest.com.au
What is Galvanic Cell? Galvanic cell, Chemistry education, Teaching Positive Electrode In Galvanic Cell Electrons can flow through an external wire and become available to do electrical work. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Indicate which electrode is the cathode and which is the anode. In. Positive Electrode In Galvanic Cell.
From mavink.com
Galvanic Cell Labeled Positive Electrode In Galvanic Cell Indicate which electrode is the positive. When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always. Positive Electrode In Galvanic Cell.
From electrochemistryh5t34.blogspot.com
We Love Electrochemistry Galvanic Cell Positive Electrode In Galvanic Cell In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Indicate which electrode is the positive. Indicate which electrode is the cathode and which is the anode. This seems reasonable as the anode is the source of. Electrons can flow through an external wire and become available to do electrical work. When the electrochemical. Positive Electrode In Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Positive Electrode In Galvanic Cell When the electrochemical cell is constructed in this fashion, a positive cell potential indicates a spontaneous reaction and that the electrons are flowing from the left. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. Electrons can flow through an external wire and become available to do electrical work. Indicate which electrode is. Positive Electrode In Galvanic Cell.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Positive Electrode In Galvanic Cell Indicate which electrode is the positive. In a galvanic (voltaic) cell, the anode is considered negative and the cathode is considered positive. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. Electrons can flow through. Positive Electrode In Galvanic Cell.
From mungfali.com
Line Notation Galvanic Cell Positive Electrode In Galvanic Cell Indicate which electrode is the cathode and which is the anode. Electrons can flow through an external wire and become available to do electrical work. With this mnemonic you can quickly remember for a galvanic cell diagram or notation that the anode is the negative electrode where oxidation takes place and is always on the left side of the. When. Positive Electrode In Galvanic Cell.