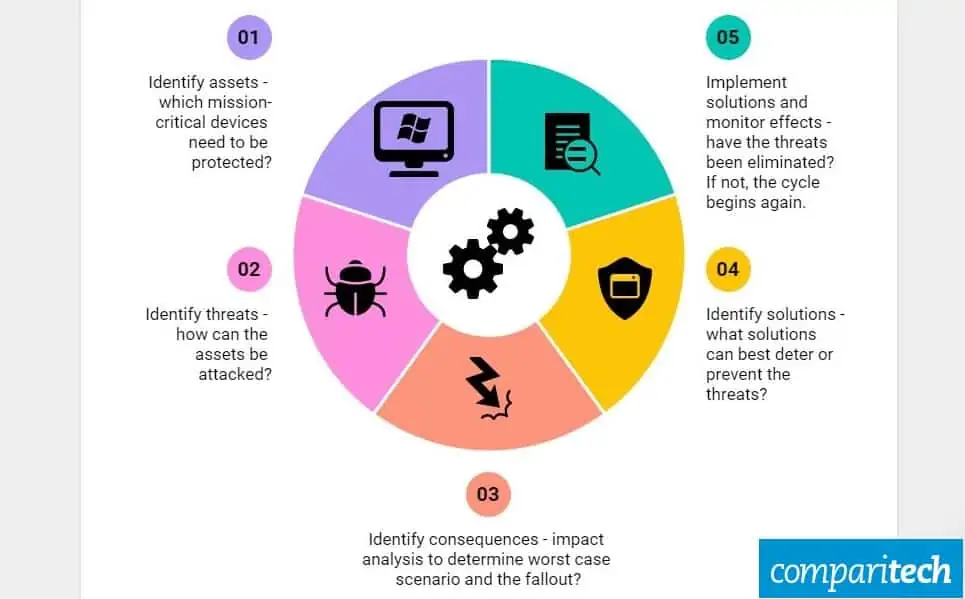
Medical Device Safety Management Using Cybersecurity Risk Analysis . As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Most medical device manufacturers have established safety risk. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. A balance needs to be achieved with safety and security, and privacy. Risk is the intersection of assets, threats, and vulnerabilities. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. In many risk assessments, this is achieved indirectly by assessing the. In medical device cybersecurity, the risk is typically associated.
from www.comparitech.com
In medical device cybersecurity, the risk is typically associated. In many risk assessments, this is achieved indirectly by assessing the. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. A balance needs to be achieved with safety and security, and privacy. Most medical device manufacturers have established safety risk. Risk is the intersection of assets, threats, and vulnerabilities. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs.
Cybersecurity risk management what is it & how to implement it in 2024
Medical Device Safety Management Using Cybersecurity Risk Analysis Most medical device manufacturers have established safety risk. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Risk is the intersection of assets, threats, and vulnerabilities. In medical device cybersecurity, the risk is typically associated. In many risk assessments, this is achieved indirectly by assessing the. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Most medical device manufacturers have established safety risk. A balance needs to be achieved with safety and security, and privacy.
From blog.cm-dm.com
Cybersecurity in medical devices Part 3 AAMI TIR572016 Software in Medical Device Safety Management Using Cybersecurity Risk Analysis Risk is the intersection of assets, threats, and vulnerabilities. A balance needs to be achieved with safety and security, and privacy. In medical device cybersecurity, the risk is typically associated. In many risk assessments, this is achieved indirectly by assessing the. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.canada.ca
Guidance Document Premarket Requirements for Medical Device Medical Device Safety Management Using Cybersecurity Risk Analysis Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Most medical device manufacturers have established safety risk. In medical device cybersecurity, the risk is typically associated. Hospital biomedical engineering teams are responsible. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.smartsheet.com
Free Cybersecurity Risk Assessment Templates Smartsheet Medical Device Safety Management Using Cybersecurity Risk Analysis Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. Most medical device manufacturers have established safety risk. In medical device cybersecurity, the risk is typically associated. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. A balance needs to be achieved with safety and security, and privacy. As. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From ar.venngage.com
الخريطة الذهنية لإطار الأمن السيبراني Venngage Medical Device Safety Management Using Cybersecurity Risk Analysis In many risk assessments, this is achieved indirectly by assessing the. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. A balance needs to be achieved with safety and security, and privacy.. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.researchgate.net
(PDF) Medical Device Safety Management Using Cybersecurity Risk Analysis Medical Device Safety Management Using Cybersecurity Risk Analysis In many risk assessments, this is achieved indirectly by assessing the. In medical device cybersecurity, the risk is typically associated. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. A balance needs to be achieved with safety and security,. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.whitecase.com
Cybersecurity Risk Top 5 strategies to build resilience White & Case LLP Medical Device Safety Management Using Cybersecurity Risk Analysis Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. In medical device cybersecurity, the risk is typically associated. Risk is the intersection of assets, threats, and vulnerabilities. Most medical device manufacturers have. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.comparitech.com
8 Best Cybersecurity Risk Management Tools 2024 (Paid & Free) Medical Device Safety Management Using Cybersecurity Risk Analysis Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Risk is the intersection of assets, threats, and vulnerabilities. Most medical device manufacturers have established safety risk. Although cybersecurity is a relatively new subject to. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Medical Device Safety Management Using Cybersecurity Risk Analysis Risk is the intersection of assets, threats, and vulnerabilities. In many risk assessments, this is achieved indirectly by assessing the. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. In medical device cybersecurity, the risk is typically associated. Most. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From bluegoatcyber.com
ISO 14971 Risk Management in Medical Device Security Blue Goat Cyber Medical Device Safety Management Using Cybersecurity Risk Analysis In medical device cybersecurity, the risk is typically associated. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Most medical device manufacturers have established safety risk. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Although cybersecurity is a relatively new subject to. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From susanshobbies.blogspot.com
Nist 800 Risk Assessment Template / Pin By Patti Gault On Safety Medical Device Safety Management Using Cybersecurity Risk Analysis Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. A balance needs to be achieved with safety and security, and privacy. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. In medical device cybersecurity, the risk is typically associated. Risk is the intersection of assets, threats, and vulnerabilities.. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.youtube.com
Conducting a cybersecurity risk assessment YouTube Medical Device Safety Management Using Cybersecurity Risk Analysis Risk is the intersection of assets, threats, and vulnerabilities. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. In many risk assessments, this is achieved indirectly by assessing the. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Most medical device manufacturers have. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From mavink.com
Risk Assessment Process Medical Device Safety Management Using Cybersecurity Risk Analysis In medical device cybersecurity, the risk is typically associated. Risk is the intersection of assets, threats, and vulnerabilities. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Although cybersecurity is a relatively new subject. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.smartsheet.com
Free Cybersecurity Risk Assessment Templates Smartsheet Medical Device Safety Management Using Cybersecurity Risk Analysis In many risk assessments, this is achieved indirectly by assessing the. A balance needs to be achieved with safety and security, and privacy. Risk is the intersection of assets, threats, and vulnerabilities. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. In medical device cybersecurity, the risk is typically associated. As part of that mission,. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.wbm.ca
Cyber Security Threat Assessment How to Manage Risk WBM Technologies Medical Device Safety Management Using Cybersecurity Risk Analysis A balance needs to be achieved with safety and security, and privacy. Most medical device manufacturers have established safety risk. In many risk assessments, this is achieved indirectly by assessing the. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Risk is the intersection of assets, threats, and. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From securityintelligence.com
Cyber Risks From the Trenches to the Boardroom Medical Device Safety Management Using Cybersecurity Risk Analysis Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. Most medical device manufacturers have established safety risk. In many risk assessments, this is achieved indirectly by assessing the. A balance needs to be achieved with safety and security, and privacy. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From onlinedegrees.und.edu
Cyber Security Risk Assessment Basics UND Online Medical Device Safety Management Using Cybersecurity Risk Analysis Most medical device manufacturers have established safety risk. In medical device cybersecurity, the risk is typically associated. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. In many risk assessments, this is achieved indirectly by assessing the. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. As part. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.smartsheet.com
Free Cybersecurity Risk Assessment Templates Smartsheet Medical Device Safety Management Using Cybersecurity Risk Analysis As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. In many risk assessments, this is achieved indirectly by. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.hayescollege.co.uk
Cyber security and risk assessment training Medical Device Safety Management Using Cybersecurity Risk Analysis Risk is the intersection of assets, threats, and vulnerabilities. A balance needs to be achieved with safety and security, and privacy. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. Hospital biomedical. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.regdesk.co
FDA on Medical Device Cybersecurity Risk Management RegDesk Medical Device Safety Management Using Cybersecurity Risk Analysis In medical device cybersecurity, the risk is typically associated. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Risk is the intersection of assets, threats, and vulnerabilities. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Most medical device manufacturers have established safety. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From corsi-dm.it
Introduction to Medical Device Safety Risk Management in Compliance Medical Device Safety Management Using Cybersecurity Risk Analysis Most medical device manufacturers have established safety risk. In many risk assessments, this is achieved indirectly by assessing the. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. A balance needs to be achieved with safety and security, and privacy. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.cyberneticgi.com
Cybersecurity Risk Management Global Intelligence Medical Device Safety Management Using Cybersecurity Risk Analysis Risk is the intersection of assets, threats, and vulnerabilities. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. In many risk assessments, this is achieved indirectly by assessing the. A balance needs to be achieved with safety and security, and privacy. In medical device cybersecurity, the risk is typically associated. As part of. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.vrogue.co
How To Perform A Cybersecurity Risk Assessment In You vrogue.co Medical Device Safety Management Using Cybersecurity Risk Analysis Risk is the intersection of assets, threats, and vulnerabilities. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. In medical device cybersecurity, the risk is typically associated. In many risk assessments, this is achieved indirectly by assessing the. Most medical device manufacturers have established safety risk. A balance. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.secjuice.com
Defining a Security Strategy WHY Medical Device Safety Management Using Cybersecurity Risk Analysis Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. A balance needs to be achieved with safety and security, and privacy. Risk is the intersection of assets, threats, and vulnerabilities. In many risk assessments, this is achieved indirectly by. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From securityboulevard.com
Risk Register Examples for Cybersecurity Leaders Security Boulevard Medical Device Safety Management Using Cybersecurity Risk Analysis As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Most medical device manufacturers have established safety risk. Risk is the intersection of assets, threats, and vulnerabilities. A balance needs to be achieved with safety and security, and privacy. Hospital biomedical engineering teams are responsible for establishing and regulating. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From strakecyber.com
Cybersecurity Risk Assessment Template for creating cybersecurity Medical Device Safety Management Using Cybersecurity Risk Analysis As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. A balance needs to be achieved with safety and security, and privacy. In medical device cybersecurity, the risk is typically associated. Most medical device manufacturers have established safety risk. Risk is the intersection of assets, threats, and vulnerabilities. In. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.smartsheet.com
Free Cybersecurity Risk Assessment Templates Smartsheet Medical Device Safety Management Using Cybersecurity Risk Analysis Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. A balance needs to be achieved with safety and security, and privacy. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From cybersecop.com
What is Cybersecurity Risk Management CyberSecOp Consulting Medical Device Safety Management Using Cybersecurity Risk Analysis Most medical device manufacturers have established safety risk. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Risk is the intersection of assets, threats, and vulnerabilities. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. A balance needs to be achieved with safety and security, and privacy. In. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.comparitech.com
Cybersecurity risk management what is it & how to implement it in 2024 Medical Device Safety Management Using Cybersecurity Risk Analysis In many risk assessments, this is achieved indirectly by assessing the. Most medical device manufacturers have established safety risk. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. As part of that mission, mdic retained debevoise & plimpton llp. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.liquidweb.com
Cybersecurity Risk Assessment Create Your First One Now Liquid Medical Device Safety Management Using Cybersecurity Risk Analysis As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Most medical device manufacturers have established safety risk. Risk is the intersection of assets, threats, and vulnerabilities. Although cybersecurity is a relatively new subject to. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.intellisoft.com.sg
Managing Cyber Security Risks (WSQ) Course in Singapore at Intellisoft Medical Device Safety Management Using Cybersecurity Risk Analysis In many risk assessments, this is achieved indirectly by assessing the. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. Most medical device manufacturers have established safety risk. Risk is the intersection of assets, threats, and vulnerabilities. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. As part. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.itsecurityguru.org
How does the board make informed decisions on cyber risk? IT Security Medical Device Safety Management Using Cybersecurity Risk Analysis As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. In medical device cybersecurity, the risk is typically associated. Most medical device manufacturers have established safety risk. A balance needs to be achieved with safety. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.nist.gov
How Vulnerable Are You To a Cyber Attack? A SelfAssessment Tool for Medical Device Safety Management Using Cybersecurity Risk Analysis Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Although cybersecurity is a relatively new subject to medical device manufacturers, safety risk management is not. In medical device cybersecurity, the risk is typically associated. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report.. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From mungfali.com
Risk Assessment Matrix For Cybersecurity Medical Device Safety Management Using Cybersecurity Risk Analysis As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Risk is the intersection of assets, threats, and vulnerabilities. In medical device cybersecurity, the risk is typically associated. Although cybersecurity is a relatively new subject. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From safety4sea.com
Infographic Ten steps to cyber security SAFETY4SEA Medical Device Safety Management Using Cybersecurity Risk Analysis Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Risk is the intersection of assets, threats, and vulnerabilities. In medical device cybersecurity, the risk is typically associated. As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. A balance needs to be achieved with. Medical Device Safety Management Using Cybersecurity Risk Analysis.
From www.mdpi.com
Risks Free FullText ContextBased and Adaptive Cybersecurity Risk Medical Device Safety Management Using Cybersecurity Risk Analysis As part of that mission, mdic retained debevoise & plimpton llp (debevoise)1 and alvarez & marsal (a&m)2 to prepare this report. Most medical device manufacturers have established safety risk. In medical device cybersecurity, the risk is typically associated. Hospital biomedical engineering teams are responsible for establishing and regulating medical equipment management programs. Risk is the intersection of assets, threats, and. Medical Device Safety Management Using Cybersecurity Risk Analysis.