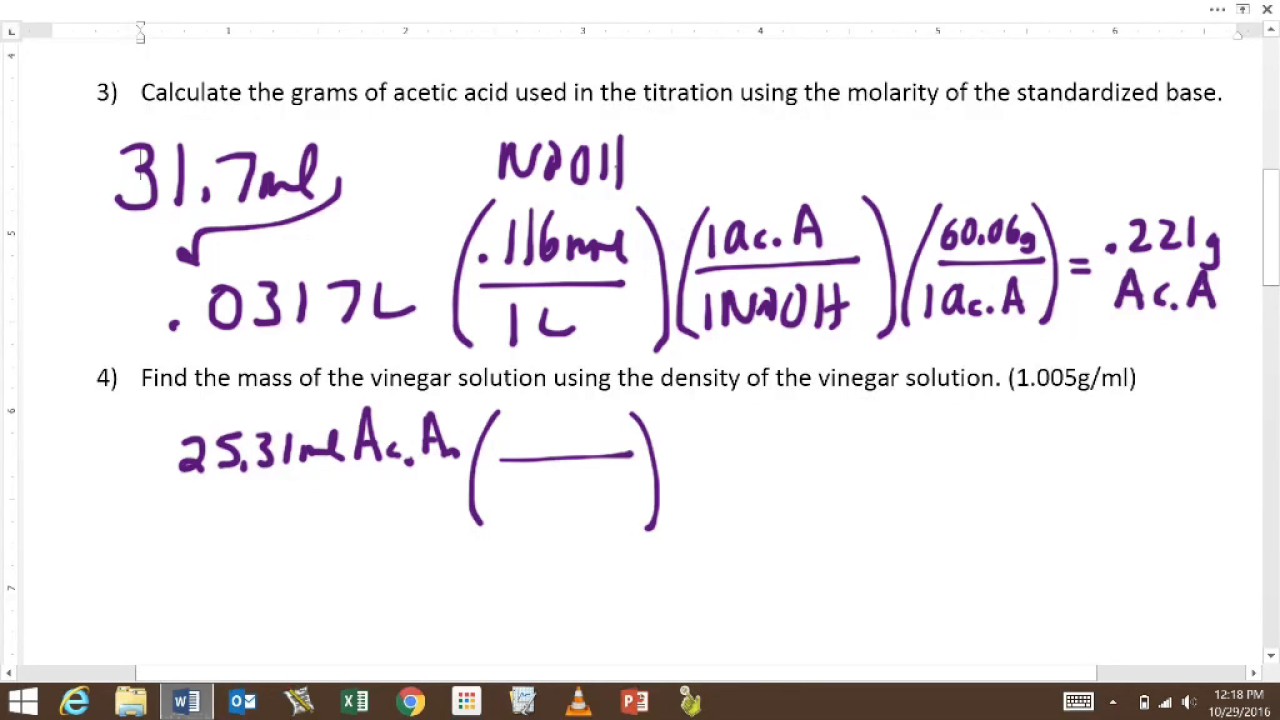
Titration Experiment Molarity . You will do this by performing a titration. 1a titration is an experimental. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): Calculate the molarity of the sulfuric acid. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. Calculate the molarity of the barium. Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4.
from cityraven.com
You will do this by performing a titration. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. 1a titration is an experimental. Calculate the molarity of the sulfuric acid. Calculate the molarity of the barium. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4.
😀 How to find the molarity of an acid. How Can PH Be Calculated From
Titration Experiment Molarity In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. You will do this by performing a titration. Calculate the molarity of the barium. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. Calculate the molarity of the sulfuric acid. 1a titration is an experimental. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution.
From es.wikihow.com
Cómo hacer una titulación (con imágenes) wikiHow Titration Experiment Molarity You will do this by performing a titration. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. Molarity = moles of acetic acid volume of vinegar. Titration Experiment Molarity.
From www.academia.edu
(PDF) Experiment 1.1 Simple AcidBase Titrations Molarity amirul Titration Experiment Molarity In this experiment, you will determine the molarity and percent composition of a weak acid using titration. Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. Calculate the molarity of the sulfuric acid. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h. Titration Experiment Molarity.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Experiment Molarity Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. Calculate the molarity of the sulfuric acid. Calculate the molarity of the barium. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. You will do this by performing a titration. In. Titration Experiment Molarity.
From www.youtube.com
Conductometric Titrations Lab YouTube Titration Experiment Molarity 1a titration is an experimental. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): In this experiment, you will determine the molarity and percent composition of a weak acid using titration. You will do this by performing a titration. Calculate the molarity of the barium. In a titration of hydrochloric acid with barium hydroxide,. Titration Experiment Molarity.
From www.youtube.com
Chem 60 Experiment 10 Part 2 Molarity of Unknown HCl Solution YouTube Titration Experiment Molarity You will do this by performing a titration. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): Calculate the molarity of the sulfuric acid. 1a titration is an experimental. Calculate the molarity of the barium. Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. In this. Titration Experiment Molarity.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Experiment Molarity The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): Calculate the molarity of the sulfuric acid. Calculate the molarity of the barium. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. In a titration of sulfuric acid. Titration Experiment Molarity.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Experiment Molarity In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or. Titration Experiment Molarity.
From aylinmeowstuart.blogspot.com
Acid Base Titration Experiment Report Titration Experiment Molarity Calculate the molarity of the barium. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. Calculate the molarity of the sulfuric acid. You will do this by performing a titration. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. In. Titration Experiment Molarity.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Titration Experiment Molarity The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): 1a titration is an experimental. Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the. Titration Experiment Molarity.
From www.chegg.com
Solved Data for Experiment 13 Determining Molarity through Titration Experiment Molarity The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. Calculate the molarity of the barium. You will do this by performing a titration. In a titration of sulfuric. Titration Experiment Molarity.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Experiment Molarity Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of. Titration Experiment Molarity.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Titration Experiment Molarity Calculate the molarity of the barium. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. 1a titration is an experimental. Calculate the molarity of the sulfuric acid. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. Molarity = moles of. Titration Experiment Molarity.
From www.studocu.com
Determining Molarity Through AcidBase Titration Lab Report Titration Experiment Molarity You will do this by performing a titration. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Calculate the molarity of the barium. Molarity = moles of acetic acid volume of. Titration Experiment Molarity.
From www.chegg.com
Solved Data for Experiment 13 Determining Molarity through Titration Experiment Molarity The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Calculate the molarity of the barium. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to. Titration Experiment Molarity.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Experiment Molarity Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. 1a titration is an experimental. Calculate the molarity of the sulfuric acid. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. In this experiment, you will. Titration Experiment Molarity.
From www.youtube.com
Experiment 7 OxidationReduction Titrations SMU Chemistry YouTube Titration Experiment Molarity In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. In a titration of sulfuric acid against sodium. Titration Experiment Molarity.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Experiment Molarity In this experiment, you will determine the molarity and percent composition of a weak acid using titration. Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. Calculate the molarity of the sulfuric acid. 1a titration is an experimental. Calculate the molarity of the barium. You will do this by performing a titration.. Titration Experiment Molarity.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Experiment Molarity Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): 1a titration is an experimental. Calculate the molarity of the sulfuric acid. In this experiment, you will determine the molarity and percent composition of a weak acid using. Titration Experiment Molarity.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Experiment Molarity In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. 1a titration is an experimental. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. Molarity = moles. Titration Experiment Molarity.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Experiment Molarity Calculate the molarity of the barium. 1a titration is an experimental. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. Calculate the molarity of the sulfuric acid. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize. Titration Experiment Molarity.
From www.youtube.com
Calculate Molarity from Titration Titration Neutralization Reaction Titration Experiment Molarity In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. In this experiment, you will determine the molarity and percent composition of a. Titration Experiment Molarity.
From www.chegg.com
Solved Experiment 17B AcidBase Titration OBJECTIVES In Titration Experiment Molarity In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. 1a titration is an experimental. The concentration of acetic acid in vinegar may. Titration Experiment Molarity.
From www.youtube.com
Molarity calculation of an acid when titrated with a base YouTube Titration Experiment Molarity In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): 1a titration. Titration Experiment Molarity.
From www.vernier.com
OxidationReduction Titrations > Experiment 19 from Investigating Titration Experiment Molarity Calculate the molarity of the barium. You will do this by performing a titration. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so. Titration Experiment Molarity.
From www.youtube.com
Titration Experiment & Calculate the Molarity of Acetic Acid in Vinegar Titration Experiment Molarity Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. In a titration of sulfuric. Titration Experiment Molarity.
From cityraven.com
😀 How to find the molarity of an acid. How Can PH Be Calculated From Titration Experiment Molarity In this experiment, you will determine the molarity and percent composition of a weak acid using titration. 1a titration is an experimental. Calculate the molarity of the barium. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. You will do this by performing. Titration Experiment Molarity.
From www.chegg.com
Solved Experiment 11, Titrations Report Sheet Titration Experiment Molarity The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): Calculate the molarity of the sulfuric acid. 1a titration is an experimental. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. In this experiment, you will determine the. Titration Experiment Molarity.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Experiment Molarity Calculate the molarity of the sulfuric acid. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. You will do this by performing a titration. In this experiment, you will determine the molarity and percent composition of a weak acid using titration. Calculate the. Titration Experiment Molarity.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Experiment Molarity Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or as a mass percent. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): You will do this by performing a titration. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75. Titration Experiment Molarity.
From www.coursehero.com
[Solved] Calculate molarity of acetic acid in vinegar solution. Record Titration Experiment Molarity Calculate the molarity of the sulfuric acid. Calculate the molarity of the barium. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. In a titration of sulfuric acid. Titration Experiment Molarity.
From www.chegg.com
Solved Lab 12 Acid Base Titration Report Part 1 Titration Experiment Molarity You will do this by performing a titration. In a titration of sulfuric acid against sodium hydroxide, 32.20 ml of 0.250 m naoh is required to neutralize 26.60 ml of h 2 so 4. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution.. Titration Experiment Molarity.
From www.chegg.com
Lab 8 Analysis of Vinegar by Titration This page Titration Experiment Molarity Calculate the molarity of the barium. You will do this by performing a titration. 1a titration is an experimental. Calculate the molarity of the sulfuric acid. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Molarity = moles of acetic acid volume of vinegar (in l) (11.1) or. Titration Experiment Molarity.
From www.scribd.com
chemistry lab report titration lab Molar Concentration Mole (Unit) Titration Experiment Molarity Calculate the molarity of the sulfuric acid. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): You will do this by performing a titration. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. 1a titration is an. Titration Experiment Molarity.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration Titration Experiment Molarity Calculate the molarity of the sulfuric acid. 1a titration is an experimental. In a titration of hydrochloric acid with barium hydroxide, 32.00 ml of 0.150 m hcl is required to neutralize 26.75 ml of the barium hydroxide solution. The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): In this experiment, you will determine the. Titration Experiment Molarity.
From www.chegg.com
Solved PART A Calculate the molarity of the NaOH solution Titration Experiment Molarity The concentration of acetic acid in vinegar may be expressed as a molarity (in mol/l): In this experiment, you will determine the molarity and percent composition of a weak acid using titration. You will do this by performing a titration. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so. Titration Experiment Molarity.