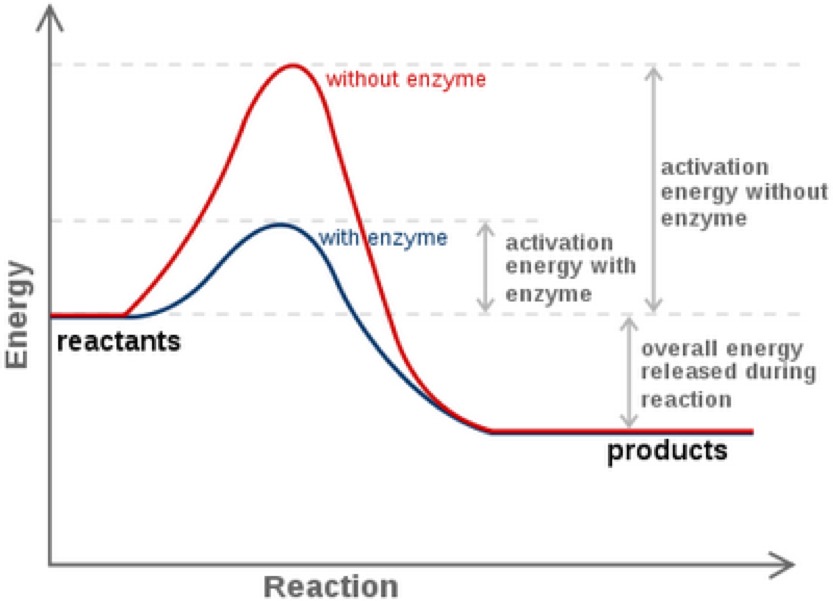
Enzyme Lower The Activation Energy Of The Reaction . One of the ways the activation energy is lowered is having the. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. The increased rate is the same in both. Enzymes lower the activation energy necessary to transform a reactant into a product. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. How do enzymes speed up biochemical reactions so dramatically? Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. For example, certain enzymes that hydrolyze atp form a covalent. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes can lower the activation energy of a chemical reaction in three ways.
from ar.inspiredpencil.com
For example, certain enzymes that hydrolyze atp form a covalent. Enzymes can lower the activation energy of a chemical reaction in three ways. The increased rate is the same in both. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. How do enzymes speed up biochemical reactions so dramatically? Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. One of the ways the activation energy is lowered is having the.
Enzymes Activation Energy
Enzyme Lower The Activation Energy Of The Reaction Enzymes lower the activation energy necessary to transform a reactant into a product. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes lower the activation energy necessary to transform a reactant into a product. One of the ways the activation energy is lowered is having the. For example, certain enzymes that hydrolyze atp form a covalent. The increased rate is the same in both. How do enzymes speed up biochemical reactions so dramatically? In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction.
From 2012books.lardbucket.org
Catalysis Enzyme Lower The Activation Energy Of The Reaction Enzymes can lower the activation energy of a chemical reaction in three ways. One of the ways the activation energy is lowered is having the. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. In some cases, enzymes lower the activation energy by directly participating in the. Enzyme Lower The Activation Energy Of The Reaction.
From www.vrogue.co
Activation Energy Definition Formula Diagram Examples vrogue.co Enzyme Lower The Activation Energy Of The Reaction Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. For example, certain enzymes that hydrolyze atp form a covalent. Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes can lower the activation energy of a chemical reaction in three ways. In some cases, enzymes lower the activation energy by directly participating. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT ENZYMES PowerPoint Presentation, free download ID6886656 Enzyme Lower The Activation Energy Of The Reaction How do enzymes speed up biochemical reactions so dramatically? The increased rate is the same in both. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can lower the activation energy of a chemical reaction in. Enzyme Lower The Activation Energy Of The Reaction.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Enzyme Lower The Activation Energy Of The Reaction Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. One of the ways the activation energy is lowered is having the. Enzymes are chemical catalysts. Enzyme Lower The Activation Energy Of The Reaction.
From www.chegg.com
Solved The graph below shows the energetics of an enzyme Enzyme Lower The Activation Energy Of The Reaction Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Enzymes lower the activation energy necessary to transform a reactant into a product. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. One of the ways the. Enzyme Lower The Activation Energy Of The Reaction.
From ar.inspiredpencil.com
Enzymes Activation Energy Enzyme Lower The Activation Energy Of The Reaction The increased rate is the same in both. Enzymes can lower the activation energy of a chemical reaction in three ways. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Enzymes (and other catalysts) act by reducing the activation energy,. Enzyme Lower The Activation Energy Of The Reaction.
From www.nagwa.com
Question Video Identifying the Activation Energy of an Enzyme Enzyme Lower The Activation Energy Of The Reaction Enzymes lower the activation energy necessary to transform a reactant into a product. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. The increased rate is the same in both. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Like. Enzyme Lower The Activation Energy Of The Reaction.
From www.genome.gov
Enzyme Enzyme Lower The Activation Energy Of The Reaction Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. How do enzymes speed up biochemical reactions so dramatically? On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes can lower the activation energy of a chemical reaction in three ways.. Enzyme Lower The Activation Energy Of The Reaction.
From www.pinterest.com
Pin on Enzyme Lower The Activation Energy Of The Reaction Enzymes lower the activation energy necessary to transform a reactant into a product. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. For example, certain enzymes that hydrolyze atp form a covalent. Enzymes can lower the activation energy of a chemical reaction in three ways. Like all catalysts, enzymes work by lowering the. Enzyme Lower The Activation Energy Of The Reaction.
From www.youtube.com
Enzyme Basics & How Do They Lower Activation Energy? YouTube Enzyme Lower The Activation Energy Of The Reaction Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. For example, certain enzymes that hydrolyze atp form a covalent.. Enzyme Lower The Activation Energy Of The Reaction.
From www.animalia-life.club
Enzyme Graph Transition State Enzyme Lower The Activation Energy Of The Reaction In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. The increased rate is the same in both. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. How do enzymes speed up biochemical reactions so dramatically? Enzymes can lower the activation energy of a chemical reaction in three. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free Enzyme Lower The Activation Energy Of The Reaction One of the ways the activation energy is lowered is having the. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. On the left is a reaction that is not catalyzed by an enzyme (red), and on the. Enzyme Lower The Activation Energy Of The Reaction.
From www.expii.com
What Are Enzymes? — Definition & Overview Expii Enzyme Lower The Activation Energy Of The Reaction How do enzymes speed up biochemical reactions so dramatically? Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. For example, certain enzymes that hydrolyze atp form a covalent. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can lower the activation energy of a chemical reaction in three. Enzyme Lower The Activation Energy Of The Reaction.
From telegra.ph
How do enzymes lower activation energy of chemical reactions Telegraph Enzyme Lower The Activation Energy Of The Reaction On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. One of the ways the activation energy. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT Endergonic and Exergonic Reactions PowerPoint Presentation ID Enzyme Lower The Activation Energy Of The Reaction Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Enzymes are chemical catalysts that accelerate chemical. Enzyme Lower The Activation Energy Of The Reaction.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Enzyme Lower The Activation Energy Of The Reaction On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. One of the ways the activation energy is lowered is having the. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can lower the activation energy of a chemical reaction. Enzyme Lower The Activation Energy Of The Reaction.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Enzyme Lower The Activation Energy Of The Reaction On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. For example, certain enzymes that hydrolyze atp form a covalent. One of the ways the activation energy is lowered is having the. How do enzymes speed up biochemical reactions so dramatically? Enzymes can lower the activation energy of. Enzyme Lower The Activation Energy Of The Reaction.
From studylib.net
Enzymes Lower Activation Energy Enzyme Lower The Activation Energy Of The Reaction Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. Enzymes lower the activation energy necessary to transform a reactant into a product. The increased rate is the same in both. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. In some cases, enzymes lower the activation energy by directly. Enzyme Lower The Activation Energy Of The Reaction.
From present5.com
Enzyme Structure classification and mechanism of action Enzyme Lower The Activation Energy Of The Reaction How do enzymes speed up biochemical reactions so dramatically? Enzymes lower the activation energy necessary to transform a reactant into a product. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. One of the ways the activation energy is lowered is having the. Enzymes can lower the activation energy of a chemical reaction in three ways.. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT Energy, Enzymes, and Biological Reactions PowerPoint Presentation Enzyme Lower The Activation Energy Of The Reaction The increased rate is the same in both. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes are chemical catalysts that accelerate chemical reactions. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1940469 Enzyme Lower The Activation Energy Of The Reaction Enzymes can lower the activation energy of a chemical reaction in three ways. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. The increased rate is the same in both. Enzymes (and other catalysts) act by reducing the activation energy, thereby. Enzyme Lower The Activation Energy Of The Reaction.
From www.linstitute.net
IB DP Biology HL复习笔记8.1.1 Metabolic Pathways翰林国际教育 Enzyme Lower The Activation Energy Of The Reaction Enzymes can lower the activation energy of a chemical reaction in three ways. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. For example, certain enzymes that hydrolyze atp form a covalent. One of the ways the activation energy is lowered is having the. Enzymes (and other. Enzyme Lower The Activation Energy Of The Reaction.
From animalia-life.club
Enzyme Activation Energy Graph Enzyme Lower The Activation Energy Of The Reaction Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes lower the activation energy necessary to transform a reactant into a product. How do enzymes speed up biochemical reactions so dramatically? Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. For example, certain enzymes that hydrolyze. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT Metabolism PowerPoint Presentation, free download ID1153506 Enzyme Lower The Activation Energy Of The Reaction Enzymes lower the activation energy necessary to transform a reactant into a product. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. In some cases, enzymes lower the activation energy by. Enzyme Lower The Activation Energy Of The Reaction.
From ar.inspiredpencil.com
Enzymes Activation Energy Enzyme Lower The Activation Energy Of The Reaction On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. The increased rate is the same in both. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. One of the ways the activation energy is lowered is having the. Enzymes lower the activation energy. Enzyme Lower The Activation Energy Of The Reaction.
From animalia-life.club
Enzyme Activation Energy Graph Enzyme Lower The Activation Energy Of The Reaction Enzymes lower the activation energy necessary to transform a reactant into a product. One of the ways the activation energy is lowered is having the. The increased rate is the same in both. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. For example, certain enzymes that hydrolyze atp form a covalent. Enzymes are. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT Enzymes Just because a reaction is spontaneous, that does not Enzyme Lower The Activation Energy Of The Reaction In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. For example, certain enzymes that hydrolyze atp form a covalent. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is. Enzyme Lower The Activation Energy Of The Reaction.
From ar.inspiredpencil.com
Enzymes Activation Energy Enzyme Lower The Activation Energy Of The Reaction The increased rate is the same in both. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes lower the activation energy necessary to transform a reactant. Enzyme Lower The Activation Energy Of The Reaction.
From thebiologs.blogspot.com
The BioLogs November 2013 Enzyme Lower The Activation Energy Of The Reaction One of the ways the activation energy is lowered is having the. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. The increased rate is the same in both. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Like all. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 Enzyme Lower The Activation Energy Of The Reaction In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Enzymes lower the activation energy necessary to transform a reactant into a product. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes (and other catalysts) act by reducing the activation energy,. Enzyme Lower The Activation Energy Of The Reaction.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation ID5736986 Enzyme Lower The Activation Energy Of The Reaction How do enzymes speed up biochemical reactions so dramatically? For example, certain enzymes that hydrolyze atp form a covalent. Enzymes can lower the activation energy of a chemical reaction in three ways. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. In some cases, enzymes lower the. Enzyme Lower The Activation Energy Of The Reaction.
From philschatz.com
Chemical Reactions · Anatomy and Physiology Enzyme Lower The Activation Energy Of The Reaction In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. One of the ways the activation energy is lowered is having the. On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes lower the activation energy necessary to transform a reactant into. Enzyme Lower The Activation Energy Of The Reaction.
From www.pinterest.com
Pin on Nursing physiology Enzyme Lower The Activation Energy Of The Reaction On the left is a reaction that is not catalyzed by an enzyme (red), and on the right is one that is. Enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering their activation energy. Enzymes lower the activation energy necessary to transform a reactant into a product. Like all catalysts, enzymes work by lowering the activation. Enzyme Lower The Activation Energy Of The Reaction.
From btccasting.weebly.com
Enzymes Lower The Activation Energy Of A Reaction btccasting Enzyme Lower The Activation Energy Of The Reaction Enzymes can lower the activation energy of a chemical reaction in three ways. Enzymes (and other catalysts) act by reducing the activation energy, thereby increasing the rate of reaction. One of the ways the activation energy is lowered is having the. Like all catalysts, enzymes work by lowering the activation energy of chemical reactions. The increased rate is the same. Enzyme Lower The Activation Energy Of The Reaction.
From www.chegg.com
Solved How Enzymes Function The graph presents three Enzyme Lower The Activation Energy Of The Reaction One of the ways the activation energy is lowered is having the. How do enzymes speed up biochemical reactions so dramatically? Enzymes can lower the activation energy of a chemical reaction in three ways. For example, certain enzymes that hydrolyze atp form a covalent. In some cases, enzymes lower the activation energy by directly participating in the chemical reaction. Enzymes. Enzyme Lower The Activation Energy Of The Reaction.