Does Water Evaporate Inside . When you’re boiling water on the stove, you’re adding heat to liquid water. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. The level of humidity in the air also plays a role in the process of evaporation. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. Relative humidity is related to the partial pressure of water vapor in the air. When liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve.
from fyokqptpd.blob.core.windows.net
At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Relative humidity is related to the partial pressure of water vapor in the air. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. When liquid water meets dry air, it is not in equilibrium; Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. The level of humidity in the air also plays a role in the process of evaporation. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere.
How Quickly Does Water Evaporate at Delores Kessler blog
Does Water Evaporate Inside On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. When liquid water meets dry air, it is not in equilibrium; Relative humidity is related to the partial pressure of water vapor in the air. When you’re boiling water on the stove, you’re adding heat to liquid water. The level of humidity in the air also plays a role in the process of evaporation.
From 9to5science.com
[Solved] Why does water evaporate at room temperature? 9to5Science Does Water Evaporate Inside If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. When you’re boiling water on. Does Water Evaporate Inside.
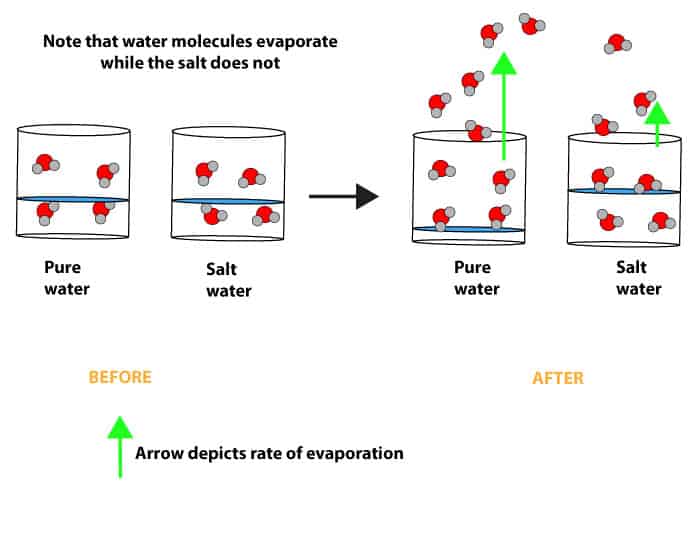
From www.slideserve.com
PPT The Water Cycle and How Humans Impact It PowerPoint Presentation Does Water Evaporate Inside Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. Evaporation occurs when energy (heat) forces the bonds that. Does Water Evaporate Inside.
From www.youtube.com
Does salt in salt water evaporate? YouTube Does Water Evaporate Inside When you’re boiling water on the stove, you’re adding heat to liquid water. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The level of humidity in the. Does Water Evaporate Inside.
From www.youtube.com
Explained !! Why Water Droplets are formed on Surface of cold bottle in Does Water Evaporate Inside On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape. Does Water Evaporate Inside.
From www.flickr.com
Does Salt Water Freeze Pictures for Emily's Science Fair p… Flickr Does Water Evaporate Inside Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. When liquid water meets dry air, it is not in equilibrium; Relative humidity is related to the partial pressure of water vapor in the air. If water evaporates at room temperature. Does Water Evaporate Inside.
From tipseri.com
How long does it take for water to evaporate at room temperature? Tipseri Does Water Evaporate Inside Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When you’re boiling water on the stove, you’re adding heat to liquid water. When liquid water meets dry air, it is not in equilibrium; The level of humidity in the air also plays a role in the process. Does Water Evaporate Inside.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Inside If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape. Does Water Evaporate Inside.
From www.snexplores.org
Explainer What are the different states of matter? Does Water Evaporate Inside If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. When you’re boiling water on the stove, you’re adding heat to liquid water. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. When liquid water meets dry air, it is not. Does Water Evaporate Inside.
From www.youtube.com
Why does water evaporate at room temp? YouTube Does Water Evaporate Inside When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. If the. Does Water Evaporate Inside.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Water Evaporate Inside Relative humidity is related to the partial pressure of water vapor in the air. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. When liquid water meets dry air, it is not in equilibrium; When the surface is exposed to sunlight, some molecules gain enough energy to escape. Does Water Evaporate Inside.
From exoojivaj.blob.core.windows.net
Does Water Evaporate When Cooking at Spencer Gilligan blog Does Water Evaporate Inside When liquid water meets dry air, it is not in equilibrium; Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. Relative humidity is related to the partial pressure of water vapor in the air. The level of humidity in the. Does Water Evaporate Inside.
From www.techexplorist.com
Light can make water evaporate without heat Does Water Evaporate Inside Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. If the partial pressure is less than the vapor pressure, then evaporation will take. Does Water Evaporate Inside.
From hxerugtas.blob.core.windows.net
Does Water Evaporate When It S Cold at Mary Austin blog Does Water Evaporate Inside Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. When liquid water meets dry air, it. Does Water Evaporate Inside.
From hxeohoirb.blob.core.windows.net
How Does Water Vapour Evaporate at Esperanza Pinto blog Does Water Evaporate Inside On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. When you’re boiling water on the stove, you’re adding heat to liquid water. The level of humidity in the air. Does Water Evaporate Inside.
From freezingflame.com
Does Water Evaporate in the Oven? Uncovering the Science Behind It Does Water Evaporate Inside Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When liquid water meets dry air, it is not in equilibrium; When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. At 100% humidity, the partial pressure is equal to the. Does Water Evaporate Inside.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube Does Water Evaporate Inside The level of humidity in the air also plays a role in the process of evaporation. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Relative humidity is related to the. Does Water Evaporate Inside.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Water Evaporate Inside If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. When liquid water meets dry air, it is not in equilibrium; At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. When the surface is exposed to sunlight, some molecules. Does Water Evaporate Inside.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Does Water Evaporate Inside If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Relative humidity is related to the partial pressure of water vapor in the air. If the partial pressure is less. Does Water Evaporate Inside.
From www.amazon.ca
Why Does Water Evaporate? All about Heat and Temperature Moore, Rob Does Water Evaporate Inside At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Relative humidity is related to the partial pressure of water vapor in the air. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. If the partial pressure. Does Water Evaporate Inside.
From hxedwgcvk.blob.core.windows.net
Does Water Evaporate From Your Body at Pamela Wittrock blog Does Water Evaporate Inside Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. The level of humidity in the air also plays a. Does Water Evaporate Inside.
From vehq.com
Does Antifreeze Evaporate Over Time? Does Water Evaporate Inside Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface. Does Water Evaporate Inside.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Inside Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. If water evaporates at room temperature because a small percentage of the molecules have enough energy to. Does Water Evaporate Inside.
From waterdefense.org
Does Chlorine Evaporate from Tap Water? (Quick Answer!) Does Water Evaporate Inside The level of humidity in the air also plays a role in the process of evaporation. Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. Water evaporates at room temperature because the molecules at the surface of the liquid have. Does Water Evaporate Inside.
From socratic.org
Through what process does water enter the atmosphere from the surface Does Water Evaporate Inside Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. When you’re boiling water on the stove, you’re adding heat to liquid water. Evaporation occurs when energy (heat) forces the bonds. Does Water Evaporate Inside.
From hxejwrxqx.blob.core.windows.net
Y Does Water Evaporate at Portia Padilla blog Does Water Evaporate Inside On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. Relative humidity is related to the partial pressure of water vapor in the air. Water evaporates at room. Does Water Evaporate Inside.
From bigtimekitchen.com
Does Water Evaporate Faster With Or Without A Lid? Does Water Evaporate Inside Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can occur at any temperature (yes, even if. When you’re boiling water on the stove, you’re adding heat to. Does Water Evaporate Inside.
From www.youtube.com
How does Water, Milk and Orange juice evaporate in 74 days? Does Water Evaporate Inside Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. When you’re boiling water on the stove, you’re adding heat to liquid water. Even at low temperatures, there are some water. Does Water Evaporate Inside.
From www.youtube.com
Does water evaporate at 60c? YouTube Does Water Evaporate Inside Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. If the partial. Does Water Evaporate Inside.
From fyocmlcvg.blob.core.windows.net
Does Water Evaporate Out Of Pool at Ronnie Hawk blog Does Water Evaporate Inside Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. The level of humidity in the air also plays a role in the process of evaporation. On. Does Water Evaporate Inside.
From www.teachoo.com
Why is Water liquid at room temperature? Give reasons Teachoo Does Water Evaporate Inside When liquid water meets dry air, it is not in equilibrium; If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. When the surface is exposed to sunlight,. Does Water Evaporate Inside.
From www.bol.com
Why Does Water Evaporate?, Rob Moore 9780521743563 Boeken Does Water Evaporate Inside The level of humidity in the air also plays a role in the process of evaporation. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Even at low temperatures, there are some. Does Water Evaporate Inside.
From bigtimekitchen.com
Does Water Evaporate Faster With Or Without A Lid? Does Water Evaporate Inside Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. The level of humidity in the air also plays a role in the process of evaporation. Water molecules evaporate off the surface until the. Does Water Evaporate Inside.
From www.youtube.com
Science Experiment Episode. 5 Water DOES NOT EVAPORATE!!! YouTube Does Water Evaporate Inside Relative humidity is related to the partial pressure of water vapor in the air. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. At 100% humidity, the partial pressure is equal to the vapor pressure, and no more water can enter the vapor phase. If the partial pressure is less than the. Does Water Evaporate Inside.
From ar.inspiredpencil.com
Can Evaporated Water Does Water Evaporate Inside When liquid water meets dry air, it is not in equilibrium; If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Relative humidity is related to the partial pressure. Does Water Evaporate Inside.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Inside If the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Evaporation occurs when energy (heat) forces the bonds that hold water molecules together to break. On a hot day, the water molecules. Does Water Evaporate Inside.