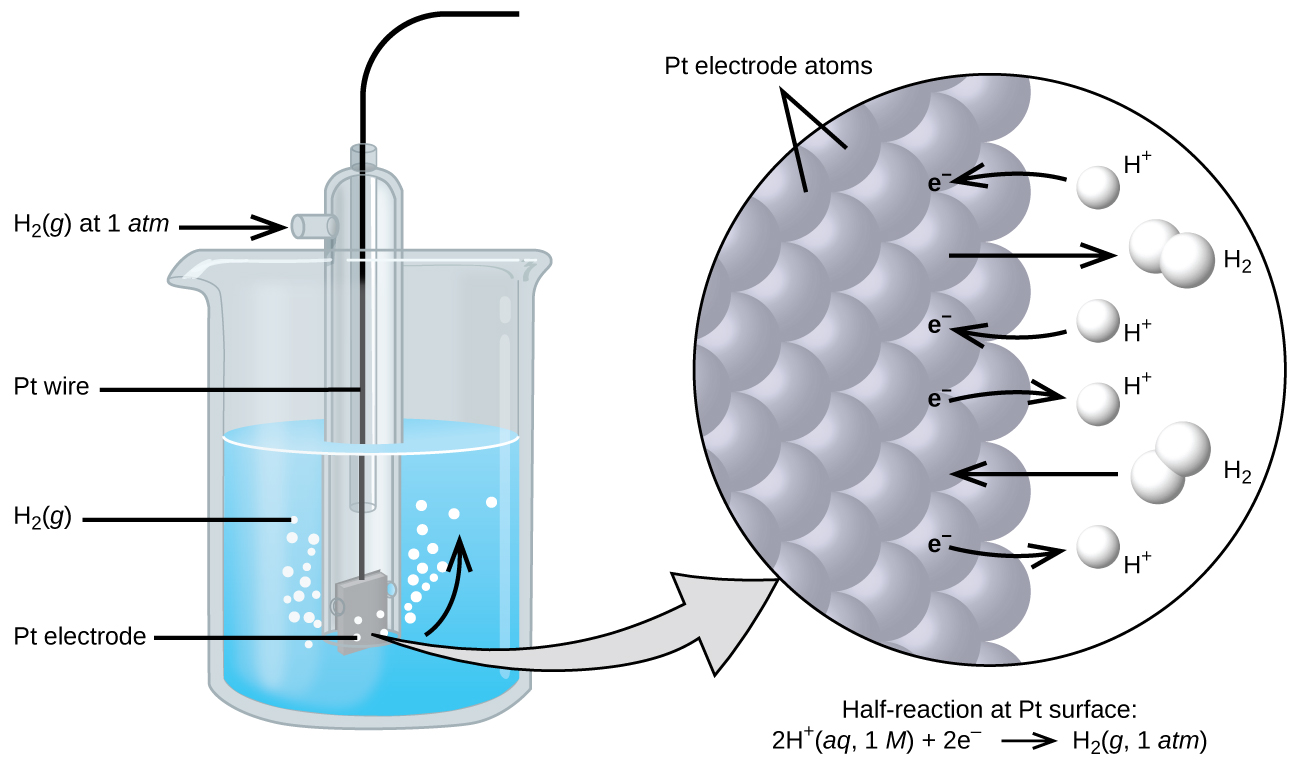
Electrode Chemical Reduction . Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell.
from chem.libretexts.org
Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under.
11.2 Standard Reduction Potential Chemistry LibreTexts
Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. Since the definition of cell. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download Electrode Chemical Reduction Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where. Electrode Chemical Reduction.
From www.researchgate.net
(a) The threeelectrode electrochemical system for the reduction of Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. Since the definition of cell. Assigning the potential of the standard hydrogen electrode (she) as zero. Electrode Chemical Reduction.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Electrode Chemical Reduction Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where. Electrode Chemical Reduction.
From www.researchgate.net
Scheme of an experimental 3 electrode electrochemical cell. Download Electrode Chemical Reduction An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction.. Electrode Chemical Reduction.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Electrode Chemical Reduction Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Since the definition of cell. An electrode reaction refers to the net oxidation or reduction process. Electrode Chemical Reduction.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Electrode Chemical Reduction An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and. Electrode Chemical Reduction.
From learningcampusstall.z21.web.core.windows.net
Consider The Following Electrochemical Cell Electrode Chemical Reduction Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and. Electrode Chemical Reduction.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrode Chemical Reduction Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs.. Electrode Chemical Reduction.
From blog.syrris.com
Electrochemistry made easy with continuous flow chemistry techniques Electrode Chemical Reduction An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as. Electrode Chemical Reduction.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Electrode Chemical Reduction Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the. Electrode Chemical Reduction.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an. Electrode Chemical Reduction.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell.. Electrode Chemical Reduction.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Electrode Chemical Reduction Since the definition of cell. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order. Electrode Chemical Reduction.
From www.youtube.com
REDOX REACTIONS AND ELECTRODE PROCESSES YouTube Electrode Chemical Reduction Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. In. Electrode Chemical Reduction.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Electrode Chemical Reduction Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order. Electrode Chemical Reduction.
From chem.libretexts.org
11.2 Standard Reduction Potential Chemistry LibreTexts Electrode Chemical Reduction Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Since. Electrode Chemical Reduction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Chemical Reduction Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. Assigning the potential of the standard hydrogen electrode (she) as zero. Electrode Chemical Reduction.
From www.nanoesclab.com
Electrochemical reduction of carbon dioxide — NanoESC Lab Electrode Chemical Reduction Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and. Electrode Chemical Reduction.
From www.researchgate.net
i Chargedischarge process of a lithiumion cell using graphite and Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Since the definition of cell. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction.. Electrode Chemical Reduction.
From classnotes.org.in
Electrochemical Series Chemistry, Class 12, Electro Chemistry Electrode Chemical Reduction An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Since the definition of cell. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction.. Electrode Chemical Reduction.
From www.ck12.org
Standard Reduction (Electrode) Potentials Example 2 ( Video Electrode Chemical Reduction Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. Assigning the potential of the standard hydrogen electrode (she) as zero. Electrode Chemical Reduction.
From www.researchgate.net
(PDF) Highly Sensitive Electrochemical Detection of DNA Hybridisation Electrode Chemical Reduction Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Since the definition of cell. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their. Electrode Chemical Reduction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Chemical Reduction Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode. Electrode Chemical Reduction.
From byjus.com
Explain the electrolysis of copper sulphate solution with the help of a Electrode Chemical Reduction Since the definition of cell. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order. Electrode Chemical Reduction.
From 2012books.lardbucket.org
Electrochemistry Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Since the definition of cell.. Electrode Chemical Reduction.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrode Chemical Reduction Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation. Electrode Chemical Reduction.
From chem.libretexts.org
11.2 Standard Electrode Potentials Chemistry LibreTexts Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. An electrode reaction refers to the net oxidation or reduction process that takes place at an. Electrode Chemical Reduction.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrode Chemical Reduction An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential. Electrode Chemical Reduction.
From www.researchgate.net
Schematic of the electrodeelectrolyte interface in electrochemical Electrode Chemical Reduction Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Since the definition of cell. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs.. Electrode Chemical Reduction.
From www.ck12.org
Standard Reduction (Electrode) Potentials Example 1 ( Video Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Electrochemical series is defined as. Electrode Chemical Reduction.
From large.stanford.edu
Electrochemical Reduction of Carbon Dioxide into Useful Fuels Electrode Chemical Reduction Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Since the definition of cell. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs.. Electrode Chemical Reduction.
From www.ck12.org
Standard Reduction (Electrode) Potentials Overview ( Video Electrode Chemical Reduction An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. Since the definition of cell. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their. Electrode Chemical Reduction.
From www.istockphoto.com
Electrode Potentials Standard Hydrogen Electrode Standard Reduction Electrode Chemical Reduction In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Since the definition of cell. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction.. Electrode Chemical Reduction.
From www.thoughtco.com
How to Define Anode and Cathode Electrode Chemical Reduction Since the definition of cell. Electrochemical series is defined as the arrangement of the elements or their ions in increasing or decreasing order of their standard electrode potential under. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. An electrode reaction refers to the net oxidation or reduction process. Electrode Chemical Reduction.
From pt.scribd.com
Standard Electrode and Reduction Potentials at 298 K Printable Sets Electrode Chemical Reduction An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard reduction. In electrochemical cells, we call the electrode where reduction occurs as the cathode and the other electrode where oxidation occurs. Electrochemical series is defined as. Electrode Chemical Reduction.