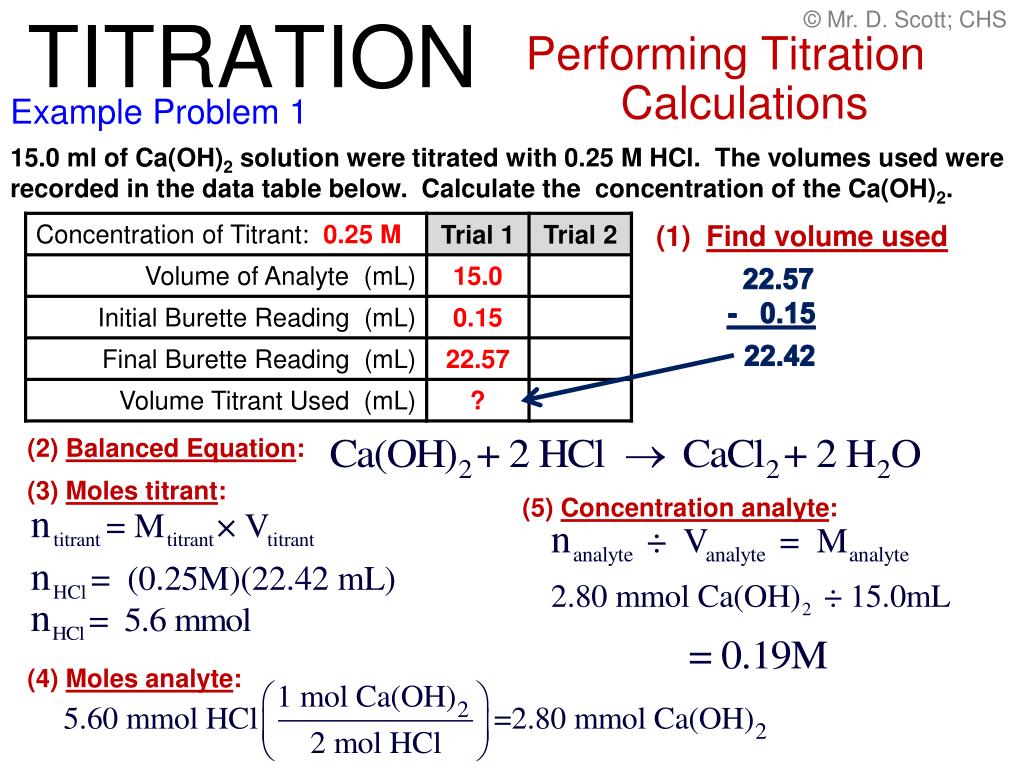
Figure Out Titration . From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The formula used in titration calculations is:. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Our titration calculator will help you never have to ask how do i calculate titrations? again. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration involves calculations to determine the concentration of the unknown substance. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. First determine the moles of \(\ce{naoh}\) in the reaction. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other.
from www.slideserve.com
Titration involves calculations to determine the concentration of the unknown substance. Our titration calculator will help you never have to ask how do i calculate titrations? again. First determine the moles of \(\ce{naoh}\) in the reaction. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The formula used in titration calculations is:. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration.
PPT TITRATION PowerPoint Presentation, free download ID1459481
Figure Out Titration A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The formula used in titration calculations is:. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Our titration calculator will help you never have to ask how do i calculate titrations? again. Titration involves calculations to determine the concentration of the unknown substance. First determine the moles of \(\ce{naoh}\) in the reaction. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Figure Out Titration A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. The formula used in titration calculations is:. First determine the moles of \(\ce{naoh}\) in the reaction. A titration calculation. Figure Out Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Figure Out Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Titration involves calculations to determine the concentration of the unknown substance. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200. Figure Out Titration.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry Figure Out Titration A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration. Figure Out Titration.
From www.youtube.com
Titration Calculations YouTube Figure Out Titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Figure Out Titration.
From www.sliderbase.com
Titration Figure Out Titration A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: A titration is a laboratory technique used to precisely. Figure Out Titration.
From labthink.netlify.app
What is titration and how does it work Figure Out Titration 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a. Figure Out Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Figure Out Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. First determine the moles of \(\ce{naoh}\) in the reaction. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a. Figure Out Titration.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Figure Out Titration First determine the moles of \(\ce{naoh}\) in the reaction. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. 0.00 ml, 15.0 ml, 25.0 ml,. Figure Out Titration.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Figure Out Titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the. Figure Out Titration.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Figure Out Titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration involves calculations to determine the concentration of the. Figure Out Titration.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Figure Out Titration First determine the moles of \(\ce{naoh}\) in the reaction. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Titration involves calculations to determine the concentration of the unknown substance. The formula used in titration calculations is:. Our titration calculator will help you never have to ask how do i calculate titrations? again. Calculate the ph for the strong acid/strong base. Figure Out Titration.
From www.science-revision.co.uk
Titrations Figure Out Titration The formula used in titration calculations is:. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Calculate. Figure Out Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Figure Out Titration 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Titration involves calculations to determine the concentration of the unknown substance. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Figure Out Titration.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Figure Out Titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Titration involves calculations to determine the concentration of the unknown substance. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Figure Out Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Figure Out Titration First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The formula used in titration calculations is:. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Titration involves calculations. Figure Out Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Figure Out Titration 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Titration involves calculations to determine the concentration of the unknown substance. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a volumetric technique in which a solution. Figure Out Titration.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Figure Out Titration A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is.. Figure Out Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME Figure Out Titration Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Figure Out Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Figure Out Titration A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration involves calculations to determine the concentration of. Figure Out Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Figure Out Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. Titration involves calculations to determine the concentration of the unknown substance. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. The formula used in. Figure Out Titration.
From www.youtube.com
titration curve pH calculations YouTube Figure Out Titration 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: From. Figure Out Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Figure Out Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. A titration is used to find an unknown concentration of a solution by reacting it. Figure Out Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Figure Out Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an. Figure Out Titration.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Figure Out Titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The formula used in titration calculations is:. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. First determine the moles of \(\ce{naoh}\) in the reaction. Titration involves calculations to determine the concentration of the unknown substance. Our titration. Figure Out Titration.
From studylib.net
How to carry out a titration Figure Out Titration A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of. Figure Out Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Figure Out Titration A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. First determine the moles of \(\ce{naoh}\) in the reaction. A. Figure Out Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Figure Out Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration calculation is a simple formula used to. Figure Out Titration.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Figure Out Titration Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. A titration is a laboratory technique used to precisely. Figure Out Titration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Figure Out Titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. First determine the moles. Figure Out Titration.
From scienceready.com.au
Titration Standard Solution, Washing, Setup HSC Chemistry Science Figure Out Titration The formula used in titration calculations is:. Titration involves calculations to determine the concentration of the unknown substance. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A. Figure Out Titration.
From www.mometrix.com
Titration Chemistry Review (Video) Figure Out Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration involves calculations to determine the concentration of the unknown substance. A titration calculation is a simple formula used to work out the concentration (in moles) of one of. Figure Out Titration.
From moodle.tau.ac.il
AcidBase Titration Curves Figure Out Titration Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is a volumetric technique in which a solution. Figure Out Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Figure Out Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. The formula used in titration calculations is:. Titration involves calculations to determine the concentration of the unknown substance. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Figure Out Titration.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Figure Out Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. First determine the moles of \(\ce{naoh}\) in the reaction. The formula used in titration calculations is:. A titration is a volumetric technique in which. Figure Out Titration.
From letitsnowglobe.co.uk
Titration procedure pdf Figure Out Titration A titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the concentration of the other. Our titration calculator will help you never have to ask how do i calculate titrations? again. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. First determine the moles of \(\ce{naoh}\). Figure Out Titration.