Standard Enthalpy Of Formation Example Problems With Answers . The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. These are worked example problems calculating the heat of formation. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. This is the physical state (solid, liquid, or gas). Three points to be made about these examples: Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. (1) every substance is shown in its standard state.
from www.chegg.com
This is the physical state (solid, liquid, or gas). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. These are worked example problems calculating the heat of formation. Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. Three points to be made about these examples: The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. (1) every substance is shown in its standard state. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f.
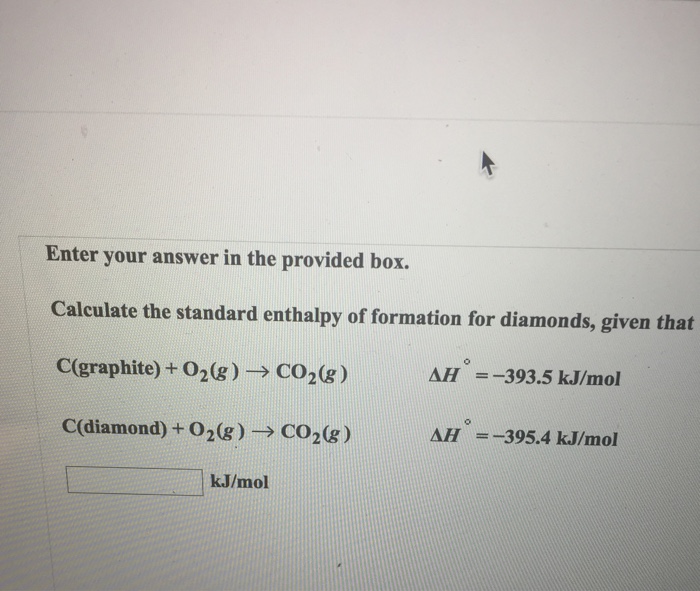
Solved Calculate the standard enthalpy of formation for
Standard Enthalpy Of Formation Example Problems With Answers Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Three points to be made about these examples: (1) every substance is shown in its standard state. This is the physical state (solid, liquid, or gas). Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. These are worked example problems calculating the heat of formation. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Example Problems With Answers Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. The symbol for the standard heat of formation (also known as the standard enthalpy of. Standard Enthalpy Of Formation Example Problems With Answers.
From www.numerade.com
SOLVED The standard enthalpy change for the following reaction is 570 Standard Enthalpy Of Formation Example Problems With Answers Three points to be made about these examples: Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. The symbol for the standard heat of formation (also known as the standard enthalpy. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
Writing Equations for Standard Enthalpy of Formation Examples YouTube Standard Enthalpy Of Formation Example Problems With Answers These are worked example problems calculating the heat of formation. Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the. Standard Enthalpy Of Formation Example Problems With Answers.
From www.studypool.com
SOLUTION Standard enthalpy of formation Studypool Standard Enthalpy Of Formation Example Problems With Answers The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of Standard Enthalpy Of Formation Example Problems With Answers The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. (1) every substance is shown in its standard state. These are worked example problems calculating the. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Example Problems With Answers The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. (1) every substance is shown in its standard state. Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate. Standard Enthalpy Of Formation Example Problems With Answers.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Example Problems With Answers These are worked example problems calculating the heat of formation. Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. Three points to be made about these examples: Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of. Standard Enthalpy Of Formation Example Problems With Answers.
From www.numerade.com
SOLVEDUsing the standard enthalpy of formation data in Appendix G Standard Enthalpy Of Formation Example Problems With Answers Three points to be made about these examples: Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. (1) every substance is shown in its standard state. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a.. Standard Enthalpy Of Formation Example Problems With Answers.
From www.numerade.com
SOLVEDFor which reaction the change of the enthalpy of the reaction Standard Enthalpy Of Formation Example Problems With Answers The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. Three points to be made about these examples: The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy. Standard Enthalpy Of Formation Example Problems With Answers.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Example Problems With Answers This is the physical state (solid, liquid, or gas). These are worked example problems calculating the heat of formation. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
Standard Enthalpies of Formation Problem (Chemistry) YouTube Standard Enthalpy Of Formation Example Problems With Answers The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. Three points to be made about these examples: (1) every substance is shown in its standard state. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of. Standard Enthalpy Of Formation Example Problems With Answers.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Example Problems With Answers Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Example Problems With Answers Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. This is the physical state (solid, liquid, or gas). Three points to be made about these examples: The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. These are worked example problems. Standard Enthalpy Of Formation Example Problems With Answers.
From askfilo.com
(9) Calculate the standard enthalpy of formation of CH3 OHin from the f.. Standard Enthalpy Of Formation Example Problems With Answers This is the physical state (solid, liquid, or gas). The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. The symbol for the standard heat of formation (also known as the standard. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
Standard Enthalpy of Formation Example 2 YouTube Standard Enthalpy Of Formation Example Problems With Answers Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. Three points to be made about these examples: The standard enthalpy of formation is a. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved Calculate the standard enthalpy of formation for Standard Enthalpy Of Formation Example Problems With Answers Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. Three points to be made about these examples: This is the physical state (solid, liquid, or gas). (1) every substance is shown in its standard state. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
Standard Enthalpy of Formation & Example Problem YouTube Standard Enthalpy Of Formation Example Problems With Answers Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. This is the physical state (solid, liquid, or gas). These are worked example problems calculating the heat of formation. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
Standard enthalpy of formation example question YouTube Standard Enthalpy Of Formation Example Problems With Answers (1) every substance is shown in its standard state. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Three points to be made about these examples: Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy.. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved 8) a) Define "standard enthalpy change of formation." Standard Enthalpy Of Formation Example Problems With Answers Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. (1) every substance is shown in its standard state. These are worked example problems calculating the heat of formation. The symbol for the standard heat of formation (also known as the standard enthalpy of formation). Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved 3.Calculate the standard enthalpy of formation of Standard Enthalpy Of Formation Example Problems With Answers This is the physical state (solid, liquid, or gas). (1) every substance is shown in its standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. These are worked example problems calculating the heat of formation. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved Calculate the standard molar enthalpy of formation Standard Enthalpy Of Formation Example Problems With Answers (1) every substance is shown in its standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. This is the physical state (solid, liquid, or gas). These are worked example problems calculating the heat of formation. Problem \(\pageindex{7}\) a sample of 0.562 g of. Standard Enthalpy Of Formation Example Problems With Answers.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation and the standard Standard Enthalpy Of Formation Example Problems With Answers Three points to be made about these examples: This is the physical state (solid, liquid, or gas). (1) every substance is shown in its standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Find the standard enthalpy of reaction (δh° rxn) for the. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved You are given the following standard enthalpies of Standard Enthalpy Of Formation Example Problems With Answers (1) every substance is shown in its standard state. These are worked example problems calculating the heat of formation. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole. Standard Enthalpy Of Formation Example Problems With Answers.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Example Problems With Answers The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Example Problems With Answers The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Enthalpy Of Formation Example Problems With Answers The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. This is the physical state (solid, liquid, or gas). Problem \(\pageindex{7}\) a sample of 0.562 g of. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
Standard heat of formation problem / Heat of formation formation Standard Enthalpy Of Formation Example Problems With Answers Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. The symbol for the standard heat of formation (also known as the standard enthalpy of formation) is δh f. Three points to be made about these examples: (1) every substance is shown in its standard. Standard Enthalpy Of Formation Example Problems With Answers.
From lessonmediavalentina.z21.web.core.windows.net
Enthalpy Practice Problems With Answers Standard Enthalpy Of Formation Example Problems With Answers The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Three points to be made about these examples: (1) every substance is shown in its standard state. These. Standard Enthalpy Of Formation Example Problems With Answers.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Enthalpy Of Formation Example Problems With Answers The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing. Standard Enthalpy Of Formation Example Problems With Answers.
From www.toppr.com
Calculate the standard enthalpy of formation of CH3 OH from the Standard Enthalpy Of Formation Example Problems With Answers These are worked example problems calculating the heat of formation. Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The symbol for the standard heat of formation. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved 13. The standard enthalpies of formation for several Standard Enthalpy Of Formation Example Problems With Answers Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. Three points to be made about these examples: The symbol for the standard heat of formation (also known as the standard enthalpy. Standard Enthalpy Of Formation Example Problems With Answers.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Example Problems With Answers The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Three points to be made about these examples: Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved Example Enthalpy Changes for a Reaction Calculate 4, Standard Enthalpy Of Formation Example Problems With Answers This is the physical state (solid, liquid, or gas). (1) every substance is shown in its standard state. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. Three points to be made about these examples: Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of. Standard Enthalpy Of Formation Example Problems With Answers.
From www.chegg.com
Solved SCH4U Standard Enthalpy of Standard Enthalpy Of Formation Example Problems With Answers Three points to be made about these examples: Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. The standard enthalpy of formation, \(δh^\circ_\ce{f}\), is the enthalpy change accompanying the formation of 1 mole of a. Problem \(\pageindex{7}\) a sample of 0.562 g of carbon is burned in oxygen in a bomb. Standard Enthalpy Of Formation Example Problems With Answers.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Example Problems With Answers These are worked example problems calculating the heat of formation. Study with quizlet and memorize flashcards containing terms like determine the standard enthalpy of reaction (δh∘rxn) given the. Find the standard enthalpy of reaction (δh° rxn) for the combustion of ethanol and calculate how much co 2 is released per kj of energy. Problem \(\pageindex{7}\) a sample of 0.562 g. Standard Enthalpy Of Formation Example Problems With Answers.