How Does Catalysts Chemical Reactions . a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a chemical. This process is called catalysis. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. what are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. With a helping hand from a catalyst, molecules that might take years to. a catalyst is some material that speeds up chemical reactions. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without.
from www.slideserve.com
a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. With a helping hand from a catalyst, molecules that might take years to. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. what are catalysts, and how do they work in terms altering the parameters of a reaction? a catalyst is some material that speeds up chemical reactions.
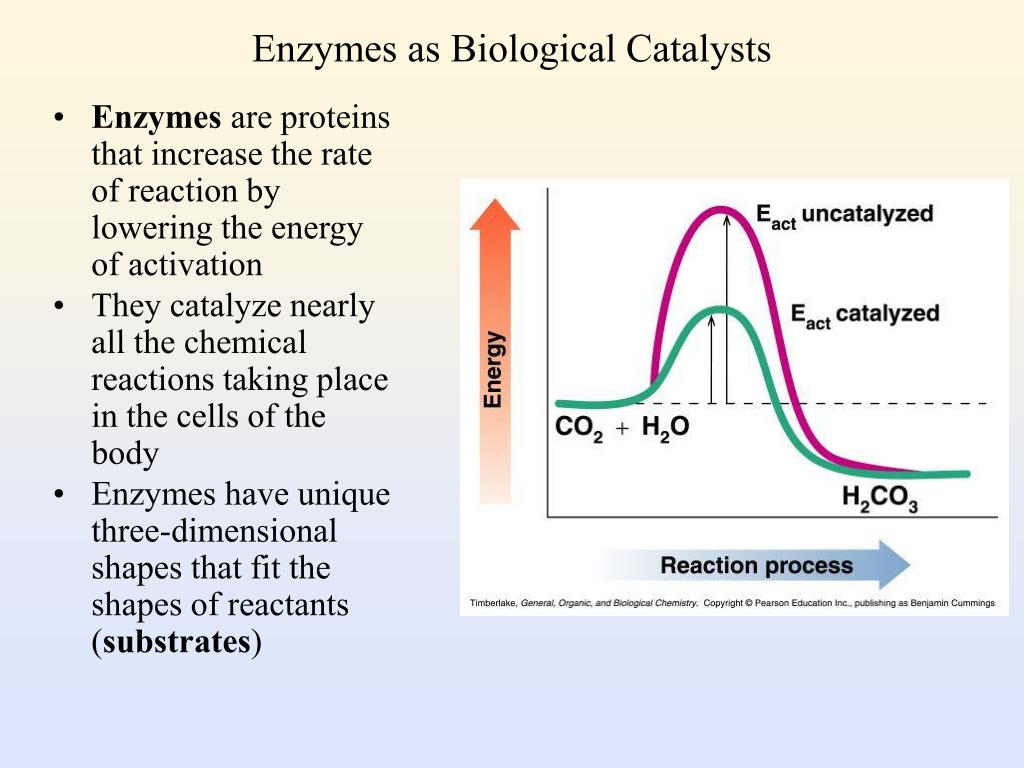
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free download ID591293
How Does Catalysts Chemical Reactions a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. a catalyst is some material that speeds up chemical reactions. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. With a helping hand from a catalyst, molecules that might take years to.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub How Does Catalysts Chemical Reactions a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. catalysis is the process that alters the rate of a. How Does Catalysts Chemical Reactions.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent How Does Catalysts Chemical Reactions catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to. This process is called catalysis. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to. How Does Catalysts Chemical Reactions.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Chemistry How Does Catalysts Chemical Reactions With a helping hand from a catalyst, molecules that might take years to. A catalyst is a chemical. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. a. How Does Catalysts Chemical Reactions.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica How Does Catalysts Chemical Reactions With a helping hand from a catalyst, molecules that might take years to. This process is called catalysis. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to. How Does Catalysts Chemical Reactions.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst. Download Scientific How Does Catalysts Chemical Reactions This process is called catalysis. A catalyst is a chemical. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. a catalyst is some material that speeds up chemical reactions. a catalyst is a chemical substance that affects the rate of a chemical reaction by. How Does Catalysts Chemical Reactions.
From www.youtube.com
Identifying catalysts in a reaction YouTube How Does Catalysts Chemical Reactions what are catalysts, and how do they work in terms altering the parameters of a reaction? This process is called catalysis. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. catalyst, in chemistry, any substance that increases the rate of a reaction without itself. How Does Catalysts Chemical Reactions.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts How Does Catalysts Chemical Reactions what are catalysts, and how do they work in terms altering the parameters of a reaction? With a helping hand from a catalyst, molecules that might take years to. A catalyst is a chemical. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. a catalyst is some material that. How Does Catalysts Chemical Reactions.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation, Chemical reactions How Does Catalysts Chemical Reactions With a helping hand from a catalyst, molecules that might take years to. what are catalysts, and how do they work in terms altering the parameters of a reaction? a catalyst is some material that speeds up chemical reactions. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A. How Does Catalysts Chemical Reactions.
From exobghamq.blob.core.windows.net
Catalysts Chemical Equation at Joy Perry blog How Does Catalysts Chemical Reactions With a helping hand from a catalyst, molecules that might take years to. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This process is called catalysis. a catalyst is some material that speeds up chemical reactions. a catalyst is a substance that speeds up a chemical reaction,. How Does Catalysts Chemical Reactions.
From 2012books.lardbucket.org
Catalysis How Does Catalysts Chemical Reactions catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. a catalyst is some material that speeds up chemical reactions. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. A catalyst is a chemical. catalyst,. How Does Catalysts Chemical Reactions.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation How Does Catalysts Chemical Reactions A catalyst is a chemical. This process is called catalysis. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. a catalyst is a chemical substance that affects. How Does Catalysts Chemical Reactions.
From exorbcrky.blob.core.windows.net
Catalyst Meaning Chem at Rick Franks blog How Does Catalysts Chemical Reactions a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst. How Does Catalysts Chemical Reactions.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube How Does Catalysts Chemical Reactions what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the. How Does Catalysts Chemical Reactions.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube How Does Catalysts Chemical Reactions catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical. what are catalysts, and how do they work in terms altering the parameters of a reaction? With a helping hand from a catalyst, molecules that might take years to. A catalyst is not consumed by the reaction. How Does Catalysts Chemical Reactions.
From www.savemyexams.com
Types of Catalyst CIE A Level Chemistry Revision Notes 2025 How Does Catalysts Chemical Reactions a catalyst is some material that speeds up chemical reactions. A catalyst is a chemical. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time.. How Does Catalysts Chemical Reactions.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation, free download ID6532825 How Does Catalysts Chemical Reactions catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. what are catalysts, and how do they work in terms altering the parameters of a reaction? With a helping hand from a catalyst, molecules that might take years to. catalyst, in chemistry, any substance that increases the rate of. How Does Catalysts Chemical Reactions.
From www.youtube.com
Catalysts Chemistry Khan Academy YouTube How Does Catalysts Chemical Reactions what are catalysts, and how do they work in terms altering the parameters of a reaction? This process is called catalysis. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is some material that speeds up chemical reactions. catalysis is the process that alters the rate of. How Does Catalysts Chemical Reactions.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) How Does Catalysts Chemical Reactions catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction. How Does Catalysts Chemical Reactions.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry How Does Catalysts Chemical Reactions catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is a chemical. what are catalysts, and how do they work in terms altering the parameters of a reaction? a. How Does Catalysts Chemical Reactions.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 How Does Catalysts Chemical Reactions a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy. How Does Catalysts Chemical Reactions.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free download ID591293 How Does Catalysts Chemical Reactions a catalyst is some material that speeds up chemical reactions. This process is called catalysis. what are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. catalyst, in chemistry, any substance that increases the. How Does Catalysts Chemical Reactions.
From ch302.cm.utexas.edu
chem1 How Does Catalysts Chemical Reactions catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. How Does Catalysts Chemical Reactions.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited How Does Catalysts Chemical Reactions With a helping hand from a catalyst, molecules that might take years to. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. a catalyst is some material that speeds up chemical reactions. A catalyst is a chemical. catalysis is the process. How Does Catalysts Chemical Reactions.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download ID501648 How Does Catalysts Chemical Reactions A catalyst is a chemical. This process is called catalysis. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. what are catalysts, and. How Does Catalysts Chemical Reactions.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 How Does Catalysts Chemical Reactions a catalyst is some material that speeds up chemical reactions. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. what are catalysts, and how do they work. How Does Catalysts Chemical Reactions.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of bind, matter 218305638 How Does Catalysts Chemical Reactions catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to. a catalyst is some material that speeds up chemical reactions. This process is called catalysis. what are catalysts, and how do they work in terms altering the parameters. How Does Catalysts Chemical Reactions.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation energy Ea of the overall How Does Catalysts Chemical Reactions a catalyst is some material that speeds up chemical reactions. what are catalysts, and how do they work in terms altering the parameters of a reaction? a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without. With a helping hand from a catalyst, molecules that. How Does Catalysts Chemical Reactions.
From www.slideshare.net
Biology 2.4 How Does Catalysts Chemical Reactions a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. a catalyst is some material that speeds up chemical reactions. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without.. How Does Catalysts Chemical Reactions.
From www.slideserve.com
PPT Basic Chemistry Concepts PowerPoint Presentation, free download ID5923586 How Does Catalysts Chemical Reactions a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. With a helping hand from a catalyst, molecules that might take years to. a. How Does Catalysts Chemical Reactions.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions And Equilibrium MCAT How Does Catalysts Chemical Reactions A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. a catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without.. How Does Catalysts Chemical Reactions.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram How Does Catalysts Chemical Reactions a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysis is the process that alters the rate of a chemical reaction under the influence of. How Does Catalysts Chemical Reactions.
From www.youtube.com
How does a CATALYST work ? YouTube How Does Catalysts Chemical Reactions what are catalysts, and how do they work in terms altering the parameters of a reaction? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. a catalyst is a substance that. How Does Catalysts Chemical Reactions.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID6634764 How Does Catalysts Chemical Reactions A catalyst is a chemical. a catalyst is some material that speeds up chemical reactions. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. a catalyst is a chemical substance. How Does Catalysts Chemical Reactions.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Does Catalysts Chemical Reactions A catalyst is a chemical. This process is called catalysis. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is some material that speeds up chemical reactions. a catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for. How Does Catalysts Chemical Reactions.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 How Does Catalysts Chemical Reactions what are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. With a helping hand from a catalyst, molecules that might take years to. catalysis is the process that alters the rate of a chemical. How Does Catalysts Chemical Reactions.