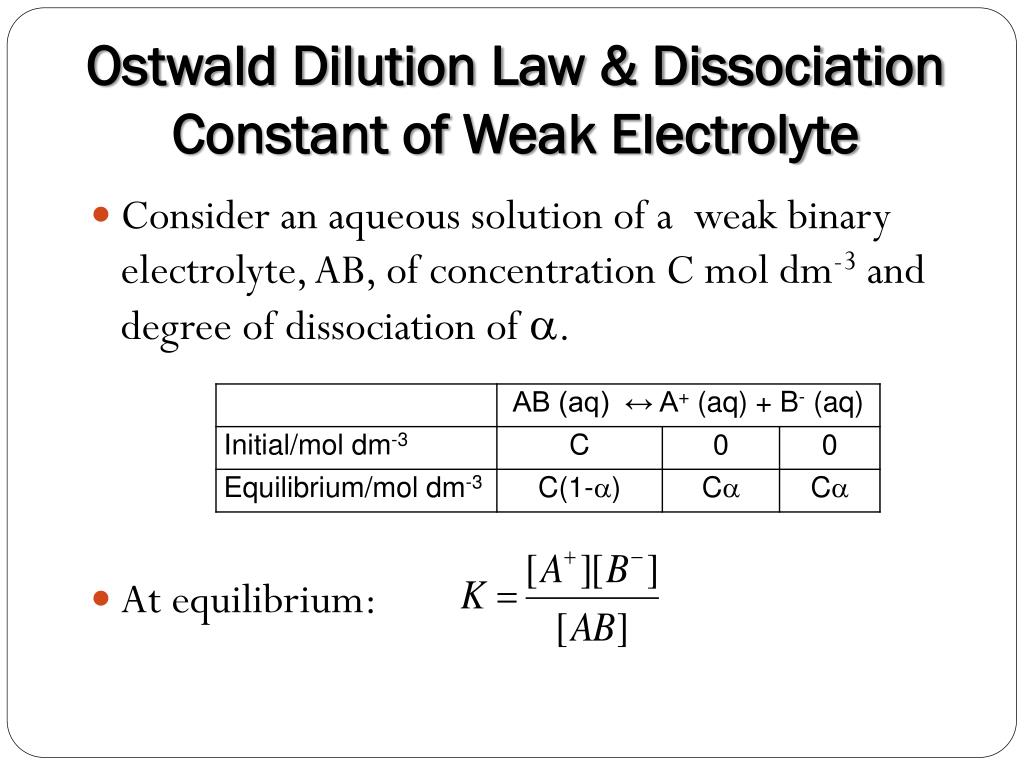
On Dilution The Solution Of An Electrolyte . on dilution, the equivalent conductivity and the molar conductivity increases. explain the distinction between ionic diffusion and ionic migration. my book says that on dilution, the degree of dissociation of an electrolyte increases. solution on diluting the solution of an electrolyte λ increases and k decreases. Define the limiting ionic conductivity, and. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. For strong electrolyte it increases due to decreased. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2.
from www.slideserve.com
on dilution, the equivalent conductivity and the molar conductivity increases. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. For strong electrolyte it increases due to decreased. solution on diluting the solution of an electrolyte λ increases and k decreases. explain the distinction between ionic diffusion and ionic migration. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. Define the limiting ionic conductivity, and. my book says that on dilution, the degree of dissociation of an electrolyte increases. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions.
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download
On Dilution The Solution Of An Electrolyte Define the limiting ionic conductivity, and. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. explain the distinction between ionic diffusion and ionic migration. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. my book says that on dilution, the degree of dissociation of an electrolyte increases. on dilution, the equivalent conductivity and the molar conductivity increases. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. Define the limiting ionic conductivity, and. For strong electrolyte it increases due to decreased. solution on diluting the solution of an electrolyte λ increases and k decreases.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation ID6829039 On Dilution The Solution Of An Electrolyte my book says that on dilution, the degree of dissociation of an electrolyte increases. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. For strong electrolyte it increases due to decreased. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10. On Dilution The Solution Of An Electrolyte.
From www.slideserve.com
PPT Making Dilutions & Electrolytes PowerPoint Presentation, free On Dilution The Solution Of An Electrolyte on dilution, the equivalent conductivity and the molar conductivity increases. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. my book. On Dilution The Solution Of An Electrolyte.
From www.youtube.com
The conductivity of an electrolytic solution decreases on dilution due On Dilution The Solution Of An Electrolyte my book says that on dilution, the degree of dissociation of an electrolyte increases. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. it revealed that ions. On Dilution The Solution Of An Electrolyte.
From byjus.com
20. Why on diluting an electrolyte it's K(specific conductivity On Dilution The Solution Of An Electrolyte Define the limiting ionic conductivity, and. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. explain the distinction between ionic diffusion and ionic migration. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure. On Dilution The Solution Of An Electrolyte.
From byjus.com
Why is it not possible to determine molar conductivity at infinite On Dilution The Solution Of An Electrolyte my book says that on dilution, the degree of dissociation of an electrolyte increases. on dilution, the equivalent conductivity and the molar conductivity increases. For strong electrolyte it increases due to decreased. solution on diluting the solution of an electrolyte λ increases and k decreases. with the accumulation of activity coefficient measurements and the search for. On Dilution The Solution Of An Electrolyte.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation On Dilution The Solution Of An Electrolyte the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. explain the distinction between ionic diffusion and ionic migration. For strong electrolyte it increases due to decreased. my book says that on dilution, the degree of dissociation of an electrolyte increases. solution on diluting the. On Dilution The Solution Of An Electrolyte.
From www.youtube.com
Ionisation of weak electrolytes and Ostwalds dilution law YouTube On Dilution The Solution Of An Electrolyte at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. explain the distinction between ionic diffusion and ionic migration. my book says. On Dilution The Solution Of An Electrolyte.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation On Dilution The Solution Of An Electrolyte at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. explain the distinction between ionic diffusion and ionic migration. my book says. On Dilution The Solution Of An Electrolyte.
From www.slideserve.com
PPT Chapter 7 Solutions of Electrolytes PowerPoint Presentation, free On Dilution The Solution Of An Electrolyte with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. on dilution, the equivalent conductivity and the molar conductivity increases. solution on diluting the solution of an electrolyte λ increases and k decreases. Define the limiting ionic conductivity, and. explain the distinction between ionic diffusion and ionic migration. . On Dilution The Solution Of An Electrolyte.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download On Dilution The Solution Of An Electrolyte explain the distinction between ionic diffusion and ionic migration. For strong electrolyte it increases due to decreased. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. my book says that on dilution, the degree of dissociation of an electrolyte increases. the effect of the concentration of ions on. On Dilution The Solution Of An Electrolyte.
From www.chegg.com
Solved Electrolytes, Concentration and Dilution of Solutions On Dilution The Solution Of An Electrolyte explain the distinction between ionic diffusion and ionic migration. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. solution on diluting the solution of an electrolyte λ increases and k decreases. my book says that on dilution, the degree of dissociation of an electrolyte. On Dilution The Solution Of An Electrolyte.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts On Dilution The Solution Of An Electrolyte my book says that on dilution, the degree of dissociation of an electrolyte increases. Define the limiting ionic conductivity, and. solution on diluting the solution of an electrolyte λ increases and k decreases. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. explain the. On Dilution The Solution Of An Electrolyte.
From www.sarthaks.com
Explain with a graph the variation of molar conductivity of a strong On Dilution The Solution Of An Electrolyte For strong electrolyte it increases due to decreased. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. on dilution, the equivalent conductivity and the molar conductivity increases. solution on diluting the solution of an electrolyte λ increases and k decreases. the effect of the concentration of ions. On Dilution The Solution Of An Electrolyte.
From exoxgkatn.blob.core.windows.net
Dilute Solution Acid at Michael Keller blog On Dilution The Solution Of An Electrolyte at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. explain the distinction between ionic diffusion and ionic migration. . On Dilution The Solution Of An Electrolyte.
From byjus.com
Why we cannot calculate the concentration of weak electrolyte at On Dilution The Solution Of An Electrolyte on dilution, the equivalent conductivity and the molar conductivity increases. my book says that on dilution, the degree of dissociation of an electrolyte increases. Define the limiting ionic conductivity, and. For strong electrolyte it increases due to decreased. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure. On Dilution The Solution Of An Electrolyte.
From kunduz.com
[ANSWERED] 5 As the dilution of an electrolyte incre... Physical On Dilution The Solution Of An Electrolyte For strong electrolyte it increases due to decreased. solution on diluting the solution of an electrolyte λ increases and k decreases. my book says that on dilution, the degree of dissociation of an electrolyte increases. Define the limiting ionic conductivity, and. explain the distinction between ionic diffusion and ionic migration. at the rather low concentration of. On Dilution The Solution Of An Electrolyte.
From www.slideserve.com
PPT CHAPTER 2 ELECTROLYTE SOLUTION PowerPoint Presentation, free On Dilution The Solution Of An Electrolyte Define the limiting ionic conductivity, and. on dilution, the equivalent conductivity and the molar conductivity increases. For strong electrolyte it increases due to decreased. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. solution on diluting the solution of an. On Dilution The Solution Of An Electrolyte.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes On Dilution The Solution Of An Electrolyte at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. Define the limiting ionic conductivity, and. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. the effect of the concentration of ions on. On Dilution The Solution Of An Electrolyte.
From kunduz.com
[ANSWERED] On increasing dilution of electrolytic so... Physical On Dilution The Solution Of An Electrolyte it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. my book says that on dilution, the degree of dissociation of an electrolyte increases. explain the distinction between ionic diffusion and ionic migration. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued,. On Dilution The Solution Of An Electrolyte.
From www.slideserve.com
PPT 24.6 The conductivities of electrolyte solutions PowerPoint On Dilution The Solution Of An Electrolyte For strong electrolyte it increases due to decreased. on dilution, the equivalent conductivity and the molar conductivity increases. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. Define. On Dilution The Solution Of An Electrolyte.
From www.slideserve.com
PPT Transport in electrolyte solutions PowerPoint Presentation ID On Dilution The Solution Of An Electrolyte with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. For strong electrolyte it increases due to decreased. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. my book says that on dilution, the degree of dissociation of. On Dilution The Solution Of An Electrolyte.
From www.youtube.com
At infinite dilution, when the dissociation of electrolyte is complete On Dilution The Solution Of An Electrolyte on dilution, the equivalent conductivity and the molar conductivity increases. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. my book says that on dilution, the degree of dissociation of an electrolyte increases. with the accumulation of activity coefficient. On Dilution The Solution Of An Electrolyte.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation On Dilution The Solution Of An Electrolyte explain the distinction between ionic diffusion and ionic migration. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. on dilution, the equivalent conductivity and the molar conductivity increases. it revealed that ions are localized in a multicoordinated ion cage. On Dilution The Solution Of An Electrolyte.
From www.youtube.com
As the dilution of an electrolyte increases CLASS 12 On Dilution The Solution Of An Electrolyte the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. with the accumulation of activity coefficient measurements and the search. On Dilution The Solution Of An Electrolyte.
From byjus.com
Consider the following statements (i) With increase in dilution On Dilution The Solution Of An Electrolyte on dilution, the equivalent conductivity and the molar conductivity increases. my book says that on dilution, the degree of dissociation of an electrolyte increases. Define the limiting ionic conductivity, and. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. with the accumulation of activity coefficient measurements and. On Dilution The Solution Of An Electrolyte.
From www.youtube.com
Explaining the electrolysis of dilute sulfuric acid H2SO4 (aq) GCSE On Dilution The Solution Of An Electrolyte solution on diluting the solution of an electrolyte λ increases and k decreases. Define the limiting ionic conductivity, and. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. For strong electrolyte it increases due to decreased. at the rather low concentration of 0.001 m, the. On Dilution The Solution Of An Electrolyte.
From www.doubtnut.com
Explain the variation of molar conductivity with concentration for str On Dilution The Solution Of An Electrolyte it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. explain the distinction between ionic diffusion and ionic migration. For strong electrolyte it increases due to decreased. Define the limiting ionic conductivity, and. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and. On Dilution The Solution Of An Electrolyte.
From www.toppr.com
Define conductivity and molar conductivity for the solution of an On Dilution The Solution Of An Electrolyte it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. solution on diluting the solution of an electrolyte λ increases and k decreases. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. the effect of the concentration of ions on. On Dilution The Solution Of An Electrolyte.
From www.youtube.com
Assertion `(A)` Equivalent conductance increase with dilution for an On Dilution The Solution Of An Electrolyte solution on diluting the solution of an electrolyte λ increases and k decreases. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in. On Dilution The Solution Of An Electrolyte.
From askfilo.com
At infinite dilution of an electrolyte, the equivalent conductances of ca.. On Dilution The Solution Of An Electrolyte Define the limiting ionic conductivity, and. solution on diluting the solution of an electrolyte λ increases and k decreases. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. on dilution, the equivalent conductivity and the molar conductivity increases. with the accumulation of activity coefficient measurements and the. On Dilution The Solution Of An Electrolyte.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL On Dilution The Solution Of An Electrolyte explain the distinction between ionic diffusion and ionic migration. For strong electrolyte it increases due to decreased. at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. on dilution, the equivalent conductivity and the molar conductivity increases. my book says. On Dilution The Solution Of An Electrolyte.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation On Dilution The Solution Of An Electrolyte with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. solution on diluting the solution of an electrolyte λ increases and k decreases. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. it revealed that ions are. On Dilution The Solution Of An Electrolyte.
From slideplayer.com
Review of Basic Concepts, Molarity, Solutions and Dilutions ppt download On Dilution The Solution Of An Electrolyte my book says that on dilution, the degree of dissociation of an electrolyte increases. Define the limiting ionic conductivity, and. on dilution, the equivalent conductivity and the molar conductivity increases. with the accumulation of activity coefficient measurements and the search for a theoretical expression ensued, it was. the effect of the concentration of ions on the. On Dilution The Solution Of An Electrolyte.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation ID297961 On Dilution The Solution Of An Electrolyte at the rather low concentration of 0.001 m, the strong electrolyte solutions conduct between 2500 and 10 000 times as much current as pure h 2. explain the distinction between ionic diffusion and ionic migration. my book says that on dilution, the degree of dissociation of an electrolyte increases. For strong electrolyte it increases due to decreased.. On Dilution The Solution Of An Electrolyte.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation ID5407668 On Dilution The Solution Of An Electrolyte For strong electrolyte it increases due to decreased. it revealed that ions are localized in a multicoordinated ion cage structure with nanoseconds in concentrated il solutions. the effect of the concentration of ions on the electrical current flowing through a solution is illustrated in figure 11.2.1 11.2. on dilution, the equivalent conductivity and the molar conductivity increases.. On Dilution The Solution Of An Electrolyte.