Define Magnetic Quantum Number . Learn how to find it and see examples. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. Find out how to derive it from the azimuthal equation and see examples of s, p, d and f subshells. The magnetic quantum number (ml). Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. It is one of the four. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. A spherical orbital has only one orientation in space. But others, such as a. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell.
from general.chemistrysteps.com
It is one of the four. Learn how to find it and see examples. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Find out how to derive it from the azimuthal equation and see examples of s, p, d and f subshells. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. The magnetic quantum number (ml). Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. But others, such as a. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field.
Quantum Numbers Chemistry Steps
Define Magnetic Quantum Number Find out how to derive it from the azimuthal equation and see examples of s, p, d and f subshells. Learn how to find it and see examples. The magnetic quantum number (ml). But others, such as a. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. It is one of the four. A spherical orbital has only one orientation in space. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. Find out how to derive it from the azimuthal equation and see examples of s, p, d and f subshells. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell.
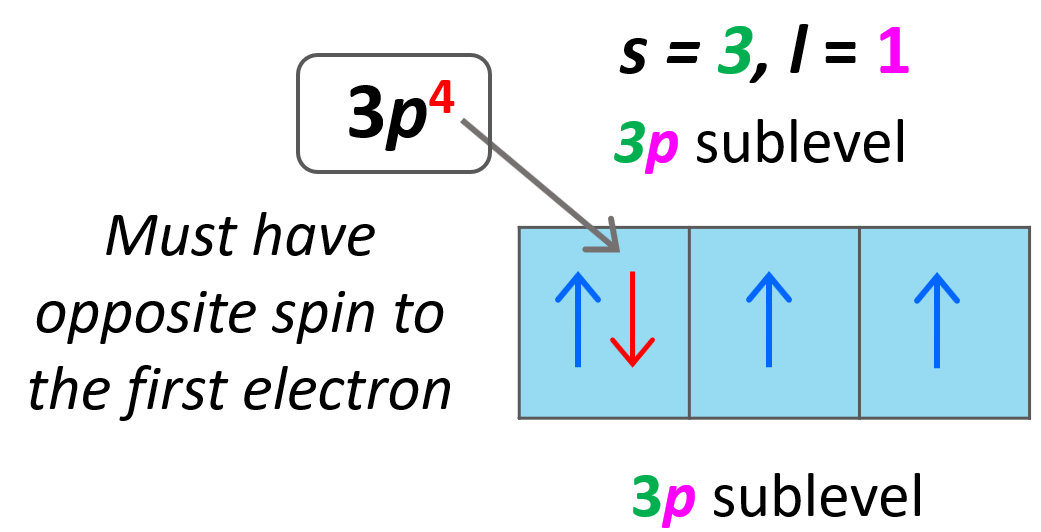
From mavink.com
Quantum Numbers Symbols Define Magnetic Quantum Number A spherical orbital has only one orientation in space. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. Learn how to find it and see examples. Learn how to use four quantum numbers (principal, angular,. Define Magnetic Quantum Number.
From testbook.com
Quantum Number Learn Definition, Formula, Applications Define Magnetic Quantum Number The magnetic quantum number (ml). The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. It is one of the four. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital. Define Magnetic Quantum Number.
From chemistnotes.com
Quantum Numbers, Types Principal, Azimuthal, and Spin Define Magnetic Quantum Number The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Find out how to derive it from the azimuthal equation and see examples of s, p, d and f. Define Magnetic Quantum Number.
From socratic.org
What are the four quantum numbers in chemistry? Socratic Define Magnetic Quantum Number But others, such as a. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum. Define Magnetic Quantum Number.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps Define Magnetic Quantum Number Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. But others, such as a. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. Learn how to find it and see examples. The magnetic quantum number (ml). Learn what magnetic quantum number is and how. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Mechanics PowerPoint Presentation, free download ID1084898 Define Magnetic Quantum Number Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. Learn how to find it and see examples. It is one of the four. But others, such as a. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. Learn how to use four quantum numbers (principal,. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Principle Quantum Numbers PowerPoint Presentation ID5519904 Define Magnetic Quantum Number Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. But others, such as a. It is one of the four. Find out how to derive it from. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation ID2135857 Define Magnetic Quantum Number The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. A spherical orbital has only one orientation in space. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. But others, such as a. Learn what magnetic quantum number is and how it determines the number and. Define Magnetic Quantum Number.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps Define Magnetic Quantum Number It is one of the four. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. But others, such as a. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. The magnetic quantum number is a quantum number that determines. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation ID2135857 Define Magnetic Quantum Number A spherical orbital has only one orientation in space. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. It is one of the four. The magnetic quantum number (ml) divides. Define Magnetic Quantum Number.
From www.geeksforgeeks.org
Quantum Numbers Principal, Azimuthal, & Spin Define Magnetic Quantum Number It is one of the four. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. Find out how to derive it from the azimuthal equation and see examples of s, p, d and f subshells. The. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID4952601 Define Magnetic Quantum Number A spherical orbital has only one orientation in space. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. It is one of the four. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. The magnetic quantum. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Electron Configuration PowerPoint Presentation, free download Define Magnetic Quantum Number Find out how to derive it from the azimuthal equation and see examples of s, p, d and f subshells. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. The magnetic quantum number (ml). Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a. Define Magnetic Quantum Number.
From study.com
Quantum Numbers on the Periodic Table Definition & Overview Lesson Define Magnetic Quantum Number The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. The magnetic quantum number (ml). Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement. Define Magnetic Quantum Number.
From www.youtube.com
Understanding Quantum Number in Quantum Mechanics YouTube Define Magnetic Quantum Number But others, such as a. It is one of the four. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn how the magnetic quantum number determines. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID2135857 Define Magnetic Quantum Number It is one of the four. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. But others, such as a. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. The magnetic quantum number (ml). Learn how to find it. Define Magnetic Quantum Number.
From www.slideserve.com
PPT AP Chemistry Unit 3 Elements PowerPoint Presentation, free Define Magnetic Quantum Number Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. It is one of the four. The magnetic quantum number (ml). The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. The magnetic quantum number is a quantum number that determines. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Unit 2 PowerPoint Presentation, free download ID298336 Define Magnetic Quantum Number Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. A spherical orbital has only one orientation in space. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. The magnetic quantum number (ml). The magnetic quantum number, often denoted as. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers and Electronic Configuration PowerPoint Define Magnetic Quantum Number Learn how to find it and see examples. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. But others, such as a. It is one of the four. A spherical orbital has only one orientation in space. Learn what magnetic quantum number is and how it determines. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 Define Magnetic Quantum Number Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. But others, such as a. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. A spherical orbital. Define Magnetic Quantum Number.
From education-portal.com
Quantum Number Definition & Example Video & Lesson Define Magnetic Quantum Number Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. But others, such as a. Learn how to find it and see examples. A spherical orbital has only one orientation in space. Find out how to derive it from the azimuthal equation and see examples of s, p, d and. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation ID2135857 Define Magnetic Quantum Number But others, such as a. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. A spherical orbital has only one orientation in space. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. Learn what magnetic quantum number is and how it determines the number. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 Define Magnetic Quantum Number It is one of the four. Learn how to find it and see examples. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. A spherical orbital has only one orientation in space. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic. Define Magnetic Quantum Number.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Define Magnetic Quantum Number But others, such as a. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. A spherical orbital has only one orientation in space. The magnetic quantum number (ml). It is. Define Magnetic Quantum Number.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry Define Magnetic Quantum Number The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. It is one of the four. Learn how to find it and see examples. But others, such as a. A spherical orbital has only one orientation in. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Arrangement of Electrons in Atoms PowerPoint Presentation, free Define Magnetic Quantum Number Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and. Define Magnetic Quantum Number.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin Define Magnetic Quantum Number The magnetic quantum number (ml). Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. Learn how to find it and see examples. Find out how to derive it from the azimuthal equation and see examples of s, p, d and f subshells. The magnetic quantum number is a quantum. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Lecture 7 Quantum Numbers and Electron Configurations PowerPoint Define Magnetic Quantum Number The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. The magnetic quantum number is a quantum number that determines the orientation of an electron's orbital in a magnetic field. Learn how to find it and see examples. A spherical orbital has only one orientation in space. It is one of the four. Find out how. Define Magnetic Quantum Number.
From byjus.com
Quantum Number Definition Schrodinger Equation Define Magnetic Quantum Number Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. It is one of the four. Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. But others, such as a. Learn how to find it and see examples. The magnetic. Define Magnetic Quantum Number.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Define Magnetic Quantum Number Find out how to derive it from the azimuthal equation and see examples of s, p, d and f subshells. Learn how to find it and see examples. But others, such as a. The magnetic quantum number (ml). Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms.. Define Magnetic Quantum Number.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Define Magnetic Quantum Number But others, such as a. It is one of the four. The magnetic quantum number (ml). Learn what magnetic quantum number is and how it determines the number and orientation of orbitals in a subshell. Learn how to find it and see examples. The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a. Define Magnetic Quantum Number.
From www.youtube.com
Quantum Numbers Azimuthal Quantum Number Quantum Spin Define Magnetic Quantum Number But others, such as a. A spherical orbital has only one orientation in space. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. Learn how to find it and see examples. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a. Define Magnetic Quantum Number.
From education-portal.com
Quantum Number Definition & Example Video & Lesson Define Magnetic Quantum Number It is one of the four. Learn how to use four quantum numbers (principal, angular, magnetic, and spin) to describe the movement and trajectories of electrons in atoms. A spherical orbital has only one orientation in space. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. Learn what magnetic. Define Magnetic Quantum Number.
From www.slideserve.com
PPT Chapter 6 Electronic Structure of Atoms PowerPoint Presentation Define Magnetic Quantum Number The magnetic quantum number, often denoted as 'm_l', specifies the orientation of an electron's orbital in a magnetic field. A spherical orbital has only one orientation in space. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. Learn what magnetic quantum number is and how it determines the number. Define Magnetic Quantum Number.
From slideplayer.com
Quantum Numbers. ppt download Define Magnetic Quantum Number But others, such as a. Learn how the magnetic quantum number determines the orientation of an electron's angular momentum in a magnetic field and its. The magnetic quantum number (ml) divides the subshell into orbitals and determines their number. Learn how to find it and see examples. A spherical orbital has only one orientation in space. Learn what magnetic quantum. Define Magnetic Quantum Number.