Boron Has A Charge Of . 93 rows this table shows the most common charges for atoms of the chemical elements. It is defined as being the charge that an atom would have if all bonds were ionic. You can use this table to predict whether an atom can bond with another atom. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). Uncombined elements have an oxidation state of 0. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: For an atom to be an ion, it wants to have full electron. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Boron has an atomic number of 5. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron would have a +3 charge.
from periodictableguide.com
Boron has an atomic number of 5. You can use this table to predict whether an atom can bond with another atom. Uncombined elements have an oxidation state of 0. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. 93 rows this table shows the most common charges for atoms of the chemical elements. Boron would have a +3 charge. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). For an atom to be an ion, it wants to have full electron. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
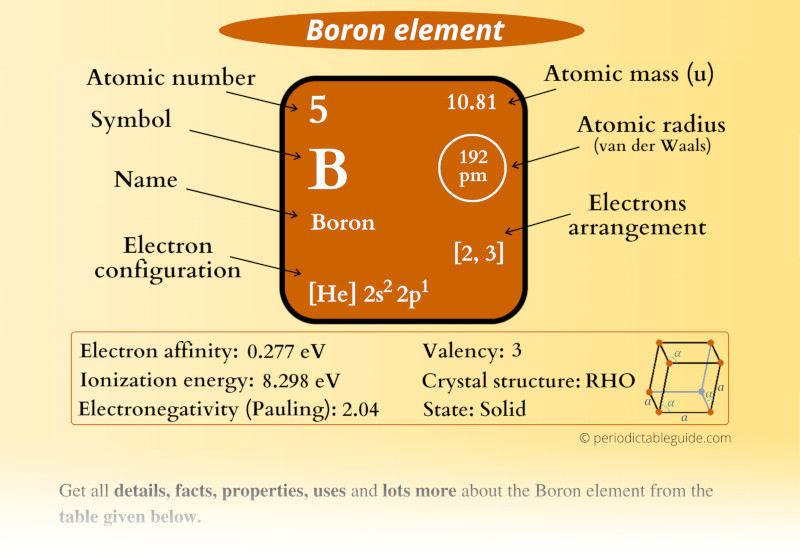
Boron (B) Periodic Table (Element Information & More)
Boron Has A Charge Of It is defined as being the charge that an atom would have if all bonds were ionic. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: For an atom to be an ion, it wants to have full electron. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows this table shows the most common charges for atoms of the chemical elements. Boron would have a +3 charge. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron has an atomic number of 5. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). You can use this table to predict whether an atom can bond with another atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
From www.examples.com
Boron (B) Definition, Preparation, Properties, Uses, Compounds Boron Has A Charge Of Boron has an atomic number of 5. It is defined as being the charge that an atom would have if all bonds were ionic. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. Uncombined. Boron Has A Charge Of.
From borates.today
Boron Electron Valence Borates Today Boron Has A Charge Of Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). Uncombined elements have an oxidation state of 0. Reduction of the oxide with magnesium or. Boron Has A Charge Of.
From manualworshipped.z14.web.core.windows.net
Boron Atom Diagram Labeled Boron Has A Charge Of Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. 93 rows this table shows the most common charges for atoms of the chemical elements. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Reduction of the oxide with magnesium or sodium gives. Boron Has A Charge Of.
From www.researchgate.net
Examples of charged or boronor siliconcontainng atom groups and their Boron Has A Charge Of 93 rows this table shows the most common charges for atoms of the chemical elements. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. For an atom to be an ion, it wants to have full electron. Uncombined elements have an oxidation state of 0. Boron has an atomic number of 5. You. Boron Has A Charge Of.
From slideplayer.com
What happens if we don’t have the same number of p+ and e? ppt download Boron Has A Charge Of 93 rows this table shows the most common charges for atoms of the chemical elements. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). Boron would have a +3 charge. Boron, chemical element that is a semimetal essential to plant. Boron Has A Charge Of.
From techiescientist.com
Boron Bohr Model Diagram, Steps To Draw Techiescientist Boron Has A Charge Of You can use this table to predict whether an atom can bond with another atom. Boron would have a +3 charge. Boron has an atomic number of 5. For an atom to be an ion, it wants to have full electron. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which. Boron Has A Charge Of.
From studylibplimsoll.z21.web.core.windows.net
What Charge Does Boron Have Boron Has A Charge Of 93 rows this table shows the most common charges for atoms of the chemical elements. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). When the atom loses or gains one or more electrons, the electric charge is generated (and. Boron Has A Charge Of.
From www.animalia-life.club
Physical Properties Of Boron Boron Has A Charge Of For an atom to be an ion, it wants to have full electron. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. It is defined as being the charge that an atom would have if all bonds were ionic. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only. Boron Has A Charge Of.
From joiqizhqn.blob.core.windows.net
Does Boron Have A Fixed Charge at Lizette Dean blog Boron Has A Charge Of Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether. Boron Has A Charge Of.
From www.benjamin-mills.com
Electron arrangements Boron Has A Charge Of You can use this table to predict whether an atom can bond with another atom. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o. Boron Has A Charge Of.
From www.researchgate.net
Examples of charged or boronor siliconcontaining atom groups and Boron Has A Charge Of For an atom to be an ion, it wants to have full electron. It is defined as being the charge that an atom would have if all bonds were ionic. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). You. Boron Has A Charge Of.
From utedzz.blogspot.com
Periodic Table Boron Atom Periodic Table Timeline Boron Has A Charge Of Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements. Boron would have a +3 charge. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: You can use this table to predict whether an atom can bond with another. Boron Has A Charge Of.
From www.animalia-life.club
Boron Bohr Diagram Boron Has A Charge Of When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). For an atom to be an ion, it wants to have full electron. Boron would have a +3 charge. Boron has an atomic number of 5. 93 rows this table shows the most common charges for atoms of the chemical. Boron Has A Charge Of.
From www.youtube.com
How to Calculate the Formal Charges for BF3 (Boron trifluoride) YouTube Boron Has A Charge Of You can use this table to predict whether an atom can bond with another atom. It is defined as being the charge that an atom would have if all bonds were ionic. Boron has an atomic number of 5. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Reduction of the oxide with. Boron Has A Charge Of.
From www.nuclear-power.com
Boron Electron Affinity Electronegativity Ionization Energy of Boron Has A Charge Of It is defined as being the charge that an atom would have if all bonds were ionic. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). 93 rows this table shows the most common charges for atoms of the chemical. Boron Has A Charge Of.
From klamosbti.blob.core.windows.net
Why Does Boron Have No Charge at Camille Snyder blog Boron Has A Charge Of 93 rows this table shows the most common charges for atoms of the chemical elements. For an atom to be an ion, it wants to have full electron. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: You can use this table to predict whether an atom can bond with another atom.. Boron Has A Charge Of.
From www.bysolar.com
How Solar Cells Work Boron Has A Charge Of For an atom to be an ion, it wants to have full electron. Boron has an atomic number of 5. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). Boron, chemical element that is a semimetal essential to plant growth. Boron Has A Charge Of.
From www.alamy.com
3d render of atom structure of boron isolated over white background Boron Has A Charge Of Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Uncombined elements have an oxidation state of 0. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Boron would have a +3 charge. Boron has an atomic number of 5. 93 rows this. Boron Has A Charge Of.
From chamotgallery.com
How many protons, neutrons and electrons does boron have? (2022) Boron Has A Charge Of Boron would have a +3 charge. Boron has an atomic number of 5. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. 93 rows. Boron Has A Charge Of.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table Boron Has A Charge Of Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements. It is defined as being the charge that an atom would have if all bonds were ionic. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: When the atom. Boron Has A Charge Of.
From www.coolgalapagos.com
Atoms the building blocks of matter Boron Has A Charge Of Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). Uncombined elements have an oxidation state of 0. Boron would have a +3 charge. You can use this table to predict whether an atom can bond with another atom. For an. Boron Has A Charge Of.
From www.animalia-life.club
Boron Element Boron Has A Charge Of For an atom to be an ion, it wants to have full electron. You can use this table to predict whether an atom can bond with another atom. Boron would have a +3 charge. Uncombined elements have an oxidation state of 0. It is defined as being the charge that an atom would have if all bonds were ionic. When. Boron Has A Charge Of.
From www.animalia-life.club
Boron Protons Neutrons Electrons Boron Has A Charge Of Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: Boron would have a +3 charge. Uncombined elements have an oxidation state of 0. 93 rows this table shows the most common charges for atoms of the chemical elements. Boron has an atomic number of 5. Boron is produced on a large scale. Boron Has A Charge Of.
From periodictable.me
How To Find The Boron Electron Configuration (B) Boron Has A Charge Of Boron would have a +3 charge. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. For an atom to be an ion, it wants to have full electron. Boron has an atomic number of 5. When. Boron Has A Charge Of.
From periodictableguide.com
Boron (B) Periodic Table (Element Information & More) Boron Has A Charge Of For an atom to be an ion, it wants to have full electron. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for. Boron Has A Charge Of.
From manuallistferraro.z13.web.core.windows.net
Electron Structure Of Boron Boron Has A Charge Of 93 rows this table shows the most common charges for atoms of the chemical elements. For an atom to be an ion, it wants to have full electron. Boron would have a +3 charge. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b. Boron Has A Charge Of.
From ar.inspiredpencil.com
Atomic Structure Of Boron Boron Has A Charge Of Boron would have a +3 charge. 93 rows this table shows the most common charges for atoms of the chemical elements. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). For an atom to be an ion, it wants to. Boron Has A Charge Of.
From www.animalia-life.club
Boron Protons Neutrons Electrons Boron Has A Charge Of Boron would have a +3 charge. 93 rows this table shows the most common charges for atoms of the chemical elements. Uncombined elements have an oxidation state of 0. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). Boron has. Boron Has A Charge Of.
From studymediapugh88.z13.web.core.windows.net
Predict Charge Of Ion From Periodic Table Boron Has A Charge Of You can use this table to predict whether an atom can bond with another atom. Boron would have a +3 charge. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). It is. Boron Has A Charge Of.
From www.sciencephoto.com
Boron, atomic structure Stock Image C018/3686 Science Photo Library Boron Has A Charge Of For an atom to be an ion, it wants to have full electron. Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: It is defined as being the charge that an atom would have if all bonds were ionic. You can use this table to predict whether an atom can bond with. Boron Has A Charge Of.
From www.animalia-life.club
Boron Protons Neutrons Electrons Boron Has A Charge Of For an atom to be an ion, it wants to have full electron. You can use this table to predict whether an atom can bond with another atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). It is defined as being the charge that an atom would have. Boron Has A Charge Of.
From www.britannica.com
boron Properties, Uses, & Facts Britannica Boron Has A Charge Of Uncombined elements have an oxidation state of 0. You can use this table to predict whether an atom can bond with another atom. It is defined as being the charge that an atom would have if all bonds were ionic. Boron, chemical element that is a semimetal essential to plant growth and of wide industrial application. Boron would have a. Boron Has A Charge Of.
From chamotgallery.com
How many protons, neutrons and electrons does boron have? (2022) Boron Has A Charge Of Reduction of the oxide with magnesium or sodium gives amorphous boron that is only about 95% pure: Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). For an atom to be an ion, it wants to have full electron. You. Boron Has A Charge Of.
From material-properties.org
Boron Periodic Table and Atomic Properties Boron Has A Charge Of When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide. Boron Has A Charge Of.
From www.numerade.com
SOLVED Calculate the formal charge of boron (B) and nitrogen (N Boron Has A Charge Of When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Uncombined elements have an oxidation state of 0. Boron is produced on a large scale by reacting borax with acid to produce boric acid [b(oh) 3], which is then dehydrated to the oxide (b 2 o 3). Boron, chemical element. Boron Has A Charge Of.