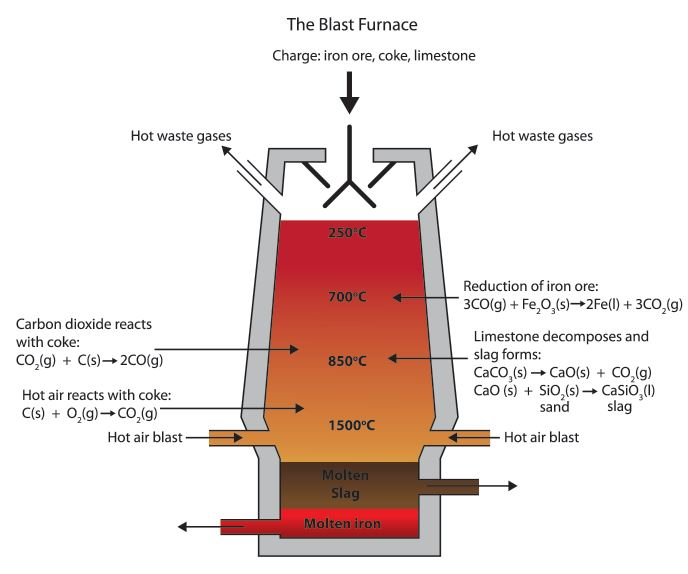
Iron (Iii) Oxide In Blast Furnace . In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. In the blast furnace, iron(iii) oxide is reduced to iron metal. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. Diagram showing the carbon extraction of iron. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. 2) in which the roasted. The furnace is filled at the top with the iron ore. The production of iron from its ore involves a redox reaction carried out in a blast furnace.
from mavink.com
Diagram showing the carbon extraction of iron. Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: The production of iron from its ore involves a redox reaction carried out in a blast furnace. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. 2) in which the roasted. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. The furnace is filled at the top with the iron ore. In the blast furnace, iron(iii) oxide is reduced to iron metal.
Blast Furnace Labelled Diagram
Iron (Iii) Oxide In Blast Furnace In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. Diagram showing the carbon extraction of iron. 2) in which the roasted. In the blast furnace, iron(iii) oxide is reduced to iron metal. The furnace is filled at the top with the iron ore. The production of iron from its ore involves a redox reaction carried out in a blast furnace.
From www.youtube.com
Extraction of Iron in the blast furnace using raw materials; coke, limestone and iron III oxide Iron (Iii) Oxide In Blast Furnace 2) in which the roasted. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. The production of iron from its ore involves a redox reaction carried out in a blast furnace. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from. Iron (Iii) Oxide In Blast Furnace.
From ironmaking-blastfurnace.blogspot.com
IRON MAKING Iron (Iii) Oxide In Blast Furnace The production of iron from its ore involves a redox reaction carried out in a blast furnace. The furnace is filled at the top with the iron ore. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. Blast furnace ironmaking is a continuous metallurgical process in which iron ore. Iron (Iii) Oxide In Blast Furnace.
From askfilo.com
3. In the blast furnace, iron (III) oxide Fe2O3 is reduced to iron Fe usi.. Iron (Iii) Oxide In Blast Furnace Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. In the blast furnace, iron(iii) oxide is reduced to iron metal. 2) in which the roasted. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in. Iron (Iii) Oxide In Blast Furnace.
From www.researchgate.net
Blast furnace process with idealized locations of iron oxides Download Scientific Diagram Iron (Iii) Oxide In Blast Furnace In the blast furnace, iron(iii) oxide is reduced to iron metal. The production of iron from its ore involves a redox reaction carried out in a blast furnace. Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. During this process, carbon monoxide, which is formed during the. Iron (Iii) Oxide In Blast Furnace.
From www.slideserve.com
PPT Metal Ores PowerPoint Presentation, free download ID5647941 Iron (Iii) Oxide In Blast Furnace Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. The furnace is filled at the top with the iron ore. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. Diagram showing the carbon extraction of iron.. Iron (Iii) Oxide In Blast Furnace.
From www.chegg.com
Solved The reduction of iron(III) oxide (Fe,0z) to pure iron Iron (Iii) Oxide In Blast Furnace Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. The furnace is filled at the top with the iron ore. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. Iron is made by reacting iron ore (iron. Iron (Iii) Oxide In Blast Furnace.
From www.znotes.org
ZNotes For Students. By Students. Iron (Iii) Oxide In Blast Furnace Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid. Iron (Iii) Oxide In Blast Furnace.
From www.blendspace.com
Extraction Of Iron Blast Furnace Lessons Blendspace Iron (Iii) Oxide In Blast Furnace Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is. Iron (Iii) Oxide In Blast Furnace.
From classnotes.org.in
Extraction of Iron Class 12, General Principles and Processes of Isolation of Elements Iron (Iii) Oxide In Blast Furnace 2) in which the roasted. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. Blast furnace ironmaking is a continuous metallurgical process. Iron (Iii) Oxide In Blast Furnace.
From mavink.com
Blast Furnace Labelled Diagram Iron (Iii) Oxide In Blast Furnace During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. 2) in which the roasted. In the blast furnace, iron(iii) oxide is reduced to iron metal. Diagram showing the carbon extraction of iron. The production of iron from its ore involves a redox reaction carried out in a blast furnace. Blast. Iron (Iii) Oxide In Blast Furnace.
From www.numerade.com
SOLVED Pure molten iron and carbon monoxide are produced in a blast furnace by the reaction of Iron (Iii) Oxide In Blast Furnace 2) in which the roasted. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. Iron, when extracted from iron ore such as haematite. Iron (Iii) Oxide In Blast Furnace.
From chem.libretexts.org
23.3 Metallurgy of Iron and Steel Chemistry LibreTexts Iron (Iii) Oxide In Blast Furnace The production of iron from its ore involves a redox reaction carried out in a blast furnace. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. In. Iron (Iii) Oxide In Blast Furnace.
From www.w3schools.blog
Iron Extraction W3schools Iron (Iii) Oxide In Blast Furnace Diagram showing the carbon extraction of iron. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. In the blast furnace, iron(iii) oxide is reduced to iron metal. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace.. Iron (Iii) Oxide In Blast Furnace.
From oneclass.com
OneClass 1. Iron is produced in a blast furnace from the reaction of iron(III) oxide with carbon... Iron (Iii) Oxide In Blast Furnace 2) in which the roasted. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: Iron is made by reacting iron ore (iron oxide and impurities), coke. Iron (Iii) Oxide In Blast Furnace.
From quizlet.com
national 5 chemistry the blast furnace Diagram Quizlet Iron (Iii) Oxide In Blast Furnace Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. The production of iron from its ore involves a redox reaction carried out. Iron (Iii) Oxide In Blast Furnace.
From www.science-revision.co.uk
moles and equations Iron (Iii) Oxide In Blast Furnace Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. 2) in which the roasted. The production of iron from its ore involves a redox reaction carried out in a blast furnace. Diagram showing the carbon extraction of iron. During this process, carbon monoxide, which. Iron (Iii) Oxide In Blast Furnace.
From www.vrogue.co
The Iron Blast Furnace Working Principle And Reaction vrogue.co Iron (Iii) Oxide In Blast Furnace During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. Diagram showing the carbon extraction of iron. In the blast furnace, iron(iii) oxide is reduced to iron metal. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii). Iron (Iii) Oxide In Blast Furnace.
From igcseandialchemistry.com
IGCSE Extraction of Metals From Ores Notes IGCSE And IAL Chemistry Iron (Iii) Oxide In Blast Furnace Diagram showing the carbon extraction of iron. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. 2) in which the roasted. During this process,. Iron (Iii) Oxide In Blast Furnace.
From www.researchgate.net
Schematic vertical crosssection of the ironmaking blast furnace and... Download Scientific Iron (Iii) Oxide In Blast Furnace Diagram showing the carbon extraction of iron. The production of iron from its ore involves a redox reaction carried out in a blast furnace. The furnace is filled at the top with the iron ore. In the blast furnace, iron(iii) oxide is reduced to iron metal. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2. Iron (Iii) Oxide In Blast Furnace.
From www.dierk-raabe.com
Metallurgical Materials Science and Alloy Design Combining direct and plasma reduction Iron (Iii) Oxide In Blast Furnace Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. The furnace is filled at the top with the iron ore. In the. Iron (Iii) Oxide In Blast Furnace.
From www.lmmgroupcn.com
Blast furnace process magnesite carbon bricks supplier,rolling mill rolls supplier,graphite Iron (Iii) Oxide In Blast Furnace The furnace is filled at the top with the iron ore. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: Diagram showing the carbon extraction of iron. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a. Iron (Iii) Oxide In Blast Furnace.
From www.learnatnoon.com
Write down the reactions taking place in different zones in the blast furnace during the Iron (Iii) Oxide In Blast Furnace Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid pig iron in a blast furnace. 2) in which the roasted. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. In the blast furnace, it is so hot. Iron (Iii) Oxide In Blast Furnace.
From www.chegg.com
Solved Blast furnaces extract pure iron from the iron(III) Iron (Iii) Oxide In Blast Furnace 2) in which the roasted. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. The production of iron from its ore involves a redox reaction carried out in a blast furnace. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is. Iron (Iii) Oxide In Blast Furnace.
From www.onlinemathlearning.com
Industrial Chemistry IGCSE Chemistry (solutions, examples, worksheets, videos) Iron (Iii) Oxide In Blast Furnace In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: 2) in which the roasted. Diagram showing the carbon extraction of iron. Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. Iron, when. Iron (Iii) Oxide In Blast Furnace.
From saylordotorg.github.io
Metallurgy Iron (Iii) Oxide In Blast Furnace Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. The furnace is filled at the top with the iron ore. Diagram showing the carbon extraction. Iron (Iii) Oxide In Blast Furnace.
From byjus.com
How is iron extracted from hematite? Iron (Iii) Oxide In Blast Furnace Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. The production of iron from its ore involves a redox reaction carried out in a blast furnace. Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the. Iron (Iii) Oxide In Blast Furnace.
From www.savemyexams.com
10.2.1 Extraction of Metals CIE IGCSE Chemistry Revision Notes 2022 Save My Exams Iron (Iii) Oxide In Blast Furnace During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. Iron, when extracted from iron ore such as haematite containing iron(iii) oxide, fe 2 o 3, in a blast furnace is called iron extraction blast. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to liquid. Iron (Iii) Oxide In Blast Furnace.
From www.tuttee.co
CHEM Extraction of Metal chemistry metal extraction blast furnace electrolysis Iron (Iii) Oxide In Blast Furnace The production of iron from its ore involves a redox reaction carried out in a blast furnace. Diagram showing the carbon extraction of iron. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: During this process, carbon monoxide, which is formed during the reaction between. Iron (Iii) Oxide In Blast Furnace.
From www.researchgate.net
Blast furnace process overview Download Scientific Diagram Iron (Iii) Oxide In Blast Furnace Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. Diagram showing the carbon extraction of iron. The furnace is filled at the top with the iron ore. 2) in which the roasted. Blast furnace ironmaking is a continuous metallurgical process in which iron ore is reduced to. Iron (Iii) Oxide In Blast Furnace.
From www.youtube.com
Extraction of Iron Making Iron in the Blast Furnace teachyourselfchemistry studyfromhome Iron (Iii) Oxide In Blast Furnace During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to. Iron (Iii) Oxide In Blast Furnace.
From ar.inspiredpencil.com
Blast Furnace Diagram Gcse Iron (Iii) Oxide In Blast Furnace Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. 2) in which the roasted. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: Iron ore (hematite), coke (an impure form of carbon),. Iron (Iii) Oxide In Blast Furnace.
From ar.inspiredpencil.com
Blast Furnace Iron Iron (Iii) Oxide In Blast Furnace In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: In the blast furnace, iron(iii) oxide is reduced to iron metal. Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. The furnace is. Iron (Iii) Oxide In Blast Furnace.
From www.w3schools.blog
Iron Extraction W3schools Iron (Iii) Oxide In Blast Furnace The furnace is filled at the top with the iron ore. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. Iron is made by reacting iron ore (iron oxide and impurities), coke (a reductant) and limestone (caco 3) in a blast furnace. In the blast furnace, it is so hot. Iron (Iii) Oxide In Blast Furnace.
From www.youtube.com
In blast furnace iron oxide is reduced by YouTube Iron (Iii) Oxide In Blast Furnace In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron (iii) oxide: During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. In the blast furnace, iron(iii) oxide is reduced to iron metal. 2) in which the roasted. Blast. Iron (Iii) Oxide In Blast Furnace.
From www.slideserve.com
PPT Blast Furnace Reactions PowerPoint Presentation ID6749162 Iron (Iii) Oxide In Blast Furnace Iron ore (hematite), coke (an impure form of carbon), and limestone are added into the top of the blast furnace. During this process, carbon monoxide, which is formed during the reaction between coke and oxygen from the air, reacts. In the blast furnace, iron(iii) oxide is reduced to iron metal. 2) in which the roasted. The production of iron from. Iron (Iii) Oxide In Blast Furnace.