Aluminum Bromide And Chlorine Gas React To Form . Sodium phosphate and calcium chloride. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. Part 4 aluminium bromide and chlorine. balancing chemical equations. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: In order to sum to zero, you. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. 2al + 3x 2 → 2alx 3 (x = cl, br and i). aluminum bromide and chlorine gas react to form aluminum chlori. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide.
from www.chegg.com
In order to sum to zero, you. balancing chemical equations. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. aluminum bromide and chlorine gas react to form aluminum chlori. 2al + 3x 2 → 2alx 3 (x = cl, br and i). Part 4 aluminium bromide and chlorine. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: Sodium phosphate and calcium chloride. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide.
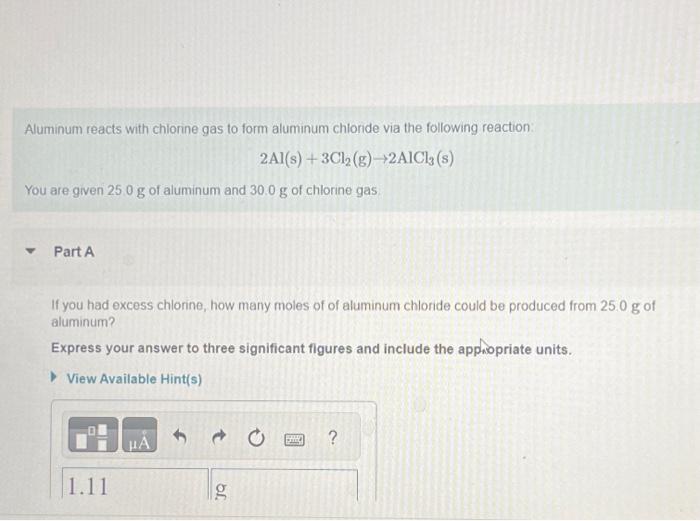
Solved Aluminum reacts with chlorine gas to form aluminum
Aluminum Bromide And Chlorine Gas React To Form aluminum bromide and chlorine gas react to form aluminum chlori. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. aluminum bromide and chlorine gas react to form aluminum chlori. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. balancing chemical equations. 2al + 3x 2 → 2alx 3 (x = cl, br and i). Part 4 aluminium bromide and chlorine. Sodium phosphate and calcium chloride. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: In order to sum to zero, you.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form Sodium phosphate and calcium chloride. 2al + 3x 2 → 2alx 3 (x = cl, br and i). Part 4 aluminium bromide and chlorine. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum. Aluminum Bromide And Chlorine Gas React To Form.
From www.coursehero.com
[Solved] Solid aluminum metal reacts with chlorine gas to produce Aluminum Bromide And Chlorine Gas React To Form albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. balancing chemical equations. aluminum bromide and chlorine gas react to form aluminum chlori. Sodium phosphate and. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVED Write the correct skeleton chemical equation for the following Aluminum Bromide And Chlorine Gas React To Form 2al + 3x 2 → 2alx 3 (x = cl, br and i). balancing chemical equations. Part 4 aluminium bromide and chlorine. Sodium phosphate and calcium chloride. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. albr3 + cl2 = br2 + alcl3 is a single. Aluminum Bromide And Chlorine Gas React To Form.
From www.coursehero.com
[Solved] Help. Part C Aluminum reacts with chlorine gas to form Aluminum Bromide And Chlorine Gas React To Form In order to sum to zero, you. Part 4 aluminium bromide and chlorine. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. balancing chemical equations. 2al. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form 2al + 3x 2 → 2alx 3 (x = cl, br and i). Part 4 aluminium bromide and chlorine. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. aluminum bromide and chlorine gas react to form aluminum chlori. albr3 + cl2 = br2 + alcl3 is. Aluminum Bromide And Chlorine Gas React To Form.
From solvedlib.com
Aluminum reacts with chlorine gas to form aluminum ch… SolvedLib Aluminum Bromide And Chlorine Gas React To Form In order to sum to zero, you. Part 4 aluminium bromide and chlorine. Sodium phosphate and calcium chloride. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide.. Aluminum Bromide And Chlorine Gas React To Form.
From www.coursehero.com
[Solved] . Aluminum reacts with chlorine gas to form aluminum chloride Aluminum Bromide And Chlorine Gas React To Form albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Sodium phosphate and calcium chloride. Part 4 aluminium bromide and chlorine. aluminum bromide and chlorine gas react to form aluminum chlori. In order to sum to zero, you. 2al + 3x 2 → 2alx 3 (x = cl,. Aluminum Bromide And Chlorine Gas React To Form.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Aluminum Bromide And Chlorine Gas React To Form Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Sodium phosphate and calcium chloride. aluminum bromide and chlorine gas react to form aluminum chlori. balancing chemical equations. albr3 +. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. In order. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVED Aluminum bromide and chlorine gas react to form aluminum Aluminum Bromide And Chlorine Gas React To Form aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. balancing. Aluminum Bromide And Chlorine Gas React To Form.
From oneclass.com
OneClass Aluminum reacts with chlorine gas to form aluminum chloride Aluminum Bromide And Chlorine Gas React To Form Sodium phosphate and calcium chloride. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. 2al + 3x 2 → 2alx 3 (x = cl, br and i).. Aluminum Bromide And Chlorine Gas React To Form.
From www.slideserve.com
PPT CLASSIFYING CHEMICAL REACTIONS PowerPoint Presentation, free Aluminum Bromide And Chlorine Gas React To Form 2al + 3x 2 → 2alx 3 (x = cl, br and i). aluminum bromide and chlorine gas react to form aluminum chlori. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. aqueous chlorine is added to a solution of aluminum bromide in a single replacement. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
3 Aluminium reacts with bromine gas to form aluminium bromide, AlBr; (a Aluminum Bromide And Chlorine Gas React To Form balancing chemical equations. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Part 4 aluminium bromide and chlorine. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. Accompanied by flames and coloured ‘smoke’, these striking. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form balancing chemical equations. Part 4 aluminium bromide and chlorine. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: albr3 + cl2 = br2 + alcl3 is a single displacement (substitution). Aluminum Bromide And Chlorine Gas React To Form.
From slideplayer.com
Chemical Reactions. ppt download Aluminum Bromide And Chlorine Gas React To Form In order to sum to zero, you. Part 4 aluminium bromide and chlorine. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. aluminum bromide and chlorine gas react to form aluminum chlori. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Solid aluminium and chlorine gas react to form Aluminum Bromide And Chlorine Gas React To Form Sodium phosphate and calcium chloride. In order to sum to zero, you. aluminum bromide and chlorine gas react to form aluminum chlori. balancing chemical equations. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. 2al + 3x 2 → 2alx 3 (x = cl, br and. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVEDAluminum and chlorine gas react to form aluminum chloride Aluminum Bromide And Chlorine Gas React To Form 2al + 3x 2 → 2alx 3 (x = cl, br and i). Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: aluminum bromide and chlorine gas react to form aluminum chlori. In order to sum to zero, you. Part 4 aluminium bromide and chlorine. albr3 + cl2 = br2 +. Aluminum Bromide And Chlorine Gas React To Form.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Aluminum Bromide And Chlorine Gas React To Form aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. 2al + 3x 2 → 2alx 3 (x = cl, br and i). In order to sum to zero, you. Part 4 aluminium bromide and chlorine. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the. Aluminum Bromide And Chlorine Gas React To Form.
From askfilo.com
Problem 3 Aluminum reacts with chlorine gas to form aluminum chloride v.. Aluminum Bromide And Chlorine Gas React To Form albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. In order to sum to zero, you. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. albr3 + cl2 = br2 + alcl3 is a single. Aluminum Bromide And Chlorine Gas React To Form.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine Aluminum Bromide And Chlorine Gas React To Form albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. aqueous. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: aluminum bromide and chlorine gas react to form aluminum chlori. albr3 + cl2 = br2 + alcl3 is a single displacement. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form Sodium phosphate and calcium chloride. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: In order to sum to zero, you. Part 4 aluminium bromide and chlorine. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. aluminum bromide and chlorine. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVED 'QUESTION 8 Aluminum will react with bromine to form aluminum Aluminum Bromide And Chlorine Gas React To Form albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. 2al + 3x 2 → 2alx 3 (x = cl, br and i). In order to sum to zero, you. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVED The reaction of aluminum with chlorine gas is shown here. Based Aluminum Bromide And Chlorine Gas React To Form 2al + 3x 2 → 2alx 3 (x = cl, br and i). albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. In order to sum to zero, you. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: balancing chemical equations. 2al + 3x 2 → 2alx 3 (x = cl, br and i). Sodium phosphate and calcium chloride. . Aluminum Bromide And Chlorine Gas React To Form.
From www.youtube.com
Reaction of Chlorine with Aluminium YouTube Aluminum Bromide And Chlorine Gas React To Form aluminum bromide and chlorine gas react to form aluminum chlori. 2al + 3x 2 → 2alx 3 (x = cl, br and i). Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: balancing chemical equations. In order to sum to zero, you. aqueous chlorine is added to a solution of. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form 2al + 3x 2 → 2alx 3 (x = cl, br and i). In order to sum to zero, you. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: Sodium phosphate and. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVED Aluminum reacts with chlorine gas to form aluminum chloride via Aluminum Bromide And Chlorine Gas React To Form albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. 2al + 3x 2 → 2alx 3 (x = cl, br and i). aluminum bromide and chlorine gas react to form aluminum chlori. Sodium phosphate and calcium chloride. Part 4 aluminium bromide and chlorine. balancing chemical equations.. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved The reaction of aluminum with chlorine gas is shown. Aluminum Bromide And Chlorine Gas React To Form Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Part 4 aluminium bromide and chlorine. 2al + 3x 2 → 2alx 3 (x = cl, br and i). Sodium phosphate and calcium. Aluminum Bromide And Chlorine Gas React To Form.
From www.chegg.com
Solved Aluminum reacts with chlorine gas to form aluminum Aluminum Bromide And Chlorine Gas React To Form albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. In order to sum to zero, you. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction. Aluminum Bromide And Chlorine Gas React To Form.
From brainly.com
Aluminum bromide and chlorine gas react to form aluminum chloride and Aluminum Bromide And Chlorine Gas React To Form 2al + 3x 2 → 2alx 3 (x = cl, br and i). Sodium phosphate and calcium chloride. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. Accompanied by flames and coloured ‘smoke’, these striking exothermic redox reactions form the solid aluminium halides: albr3 + cl =. Aluminum Bromide And Chlorine Gas React To Form.
From dxovypfff.blob.core.windows.net
Aluminum Bromide And Chlorine Gas Formula at Ashley Larkin blog Aluminum Bromide And Chlorine Gas React To Form balancing chemical equations. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Sodium phosphate and calcium chloride. In order to sum to zero, you. Accompanied by. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVED shou vour work Use the correct number of significant figures Aluminum Bromide And Chlorine Gas React To Form 2al + 3x 2 → 2alx 3 (x = cl, br and i). Part 4 aluminium bromide and chlorine. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Sodium phosphate and calcium chloride. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVEDPart III Mass Relationships in Chemical Reactions (Stoichiometry Aluminum Bromide And Chlorine Gas React To Form balancing chemical equations. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. aqueous chlorine is added to a solution of aluminum bromide in a single replacement reaction which forms no precipitate and. Part 4 aluminium bromide and chlorine. 2al + 3x 2 → 2alx 3 (x. Aluminum Bromide And Chlorine Gas React To Form.
From www.numerade.com
SOLVED Aluminum reacts with chlorine gas to produce aluminum chloride Aluminum Bromide And Chlorine Gas React To Form balancing chemical equations. Sodium phosphate and calcium chloride. In order to sum to zero, you. albr3 + cl2 = br2 + alcl3 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. albr3 + cl = alcl3 + br2 is a single displacement (substitution) reaction where two moles of aqueous aluminum bromide. Part 4. Aluminum Bromide And Chlorine Gas React To Form.