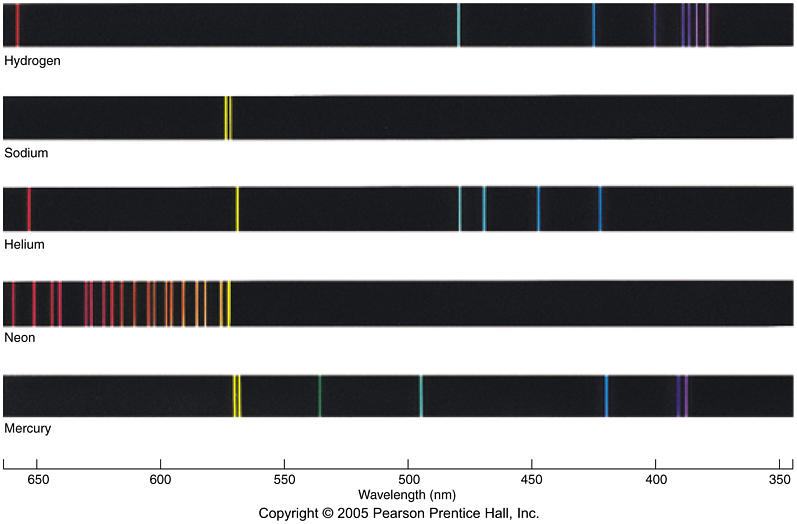
Emission Spectra Helium Lines . A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. The emission spectra of various atoms. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. The figure below shows the. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Helium (he) strong lines of helium ( he ) intensity. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. When hydrogen gas is placed.
from www.chemhume.co.uk
The figure below shows the. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emission spectra of various atoms. Helium (he) strong lines of helium ( he ) intensity. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms.
Line emission spectra
Emission Spectra Helium Lines The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Helium (he) strong lines of helium ( he ) intensity. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emission spectra of various atoms. When hydrogen gas is placed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. The figure below shows the. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all.
From www.astronoo.com
Spectroscopy — Astronoo Emission Spectra Helium Lines Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Helium (he) strong lines of helium ( he ) intensity. A matching. Emission Spectra Helium Lines.
From srkjpxyrfzubc.blogspot.com
Helium Emission Spectrum, Hydrogen Fusion and the Emission Spectrum A Emission Spectra Helium Lines A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. Helium (he) strong lines of helium ( he ) intensity. The 12 lines of the visible helium spectrum correspond to wavelengths of. Emission Spectra Helium Lines.
From www.slideserve.com
PPT Mon 9/24 and Tues 9/25 PowerPoint Presentation, free download Emission Spectra Helium Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectra of various atoms. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. Helium (he) strong lines of helium. Emission Spectra Helium Lines.
From mungfali.com
Atomic Emission Spectrum Of Elements Emission Spectra Helium Lines The emission spectra of various atoms. Helium (he) strong lines of helium ( he ) intensity. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. The figure below shows the. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. When hydrogen gas is placed. Atoms of individual. Emission Spectra Helium Lines.
From fphoto.photoshelter.com
science physics waves spectrum analysis Fundamental Emission Spectra Helium Lines Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. When hydrogen gas is placed. The figure below shows the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An atomic emission. Emission Spectra Helium Lines.
From www.slideserve.com
PPT Light Emission PowerPoint Presentation, free download ID5593597 Emission Spectra Helium Lines The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Helium (he) strong lines of helium ( he ) intensity. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Emission Spectra Helium Lines.
From pixels.com
Hydrogen And Helium Spectra Photograph by Carlos Clarivan Pixels Emission Spectra Helium Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When hydrogen gas is placed. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. The figure below shows the. The 12. Emission Spectra Helium Lines.
From ar.inspiredpencil.com
Helium Light Spectrum Emission Spectra Helium Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. The figure below shows the. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum. Emission Spectra Helium Lines.
From heliumoseiha.blogspot.com
Helium Helium Emission Spectrum Emission Spectra Helium Lines Helium (he) strong lines of helium ( he ) intensity. When hydrogen gas is placed. The figure below shows the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,.. Emission Spectra Helium Lines.
From heliumoseiha.blogspot.com
Helium Helium Spectral Lines Emission Spectra Helium Lines The emission spectra of various atoms. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. When hydrogen gas is placed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum (or line spectrum) of a. Emission Spectra Helium Lines.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra Helium Lines The figure below shows the. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen. Emission Spectra Helium Lines.
From www.chegg.com
Solved which photon of light in the Helium Emission Spectrum Emission Spectra Helium Lines Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. Helium (he) strong lines of helium ( he ) intensity. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. The figure below shows the. An atomic emission spectrum is the pattern. Emission Spectra Helium Lines.
From heliumoseiha.blogspot.com
Helium Helium Emission Spectrum Emission Spectra Helium Lines The figure below shows the. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atoms of individual elements emit light at. Emission Spectra Helium Lines.
From xmphysics.com
17.3.2 Emission Spectrum xmPhysics Emission Spectra Helium Lines The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Atoms of individual elements emit light at. Emission Spectra Helium Lines.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Helium Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emission spectra of various atoms. Atoms. Emission Spectra Helium Lines.
From www.slideserve.com
PPT The Helium Story PowerPoint Presentation, free download ID5841399 Emission Spectra Helium Lines A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. Helium (he) strong lines of helium ( he ) intensity. The emission spectra of various atoms. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. An atomic emission spectrum is the pattern of lines formed when light passes. Emission Spectra Helium Lines.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectra Helium Lines A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. When hydrogen gas is placed. The figure below shows the. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. Helium (he) strong lines of helium ( he ) intensity. Atoms of individual elements emit light at only specific. Emission Spectra Helium Lines.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Emission Spectra Helium Lines The emission spectra of various atoms. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. Helium (he) strong lines of helium ( he ) intensity. The figure below shows the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectra Helium Lines.
From www.esa.int
ESA Absorption and emission spectra of various elements Emission Spectra Helium Lines The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the. Emission Spectra Helium Lines.
From www.alamy.com
HHeHg emission spectra. Graphical representation of the emission Emission Spectra Helium Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Helium (he) strong lines of helium ( he ) intensity. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. The figure below shows the. The. Emission Spectra Helium Lines.
From heliumoseiha.blogspot.com
Helium Helium Emission Spectrum Emission Spectra Helium Lines The figure below shows the. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. The 12 lines of the visible helium spectrum correspond to wavelengths. Emission Spectra Helium Lines.
From www.coursehero.com
[Solved] 3) The spectrum of the unknown mixture is a mixture of two Emission Spectra Helium Lines Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The emission spectra of various atoms. When hydrogen gas is placed. The 12. Emission Spectra Helium Lines.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Emission Spectra Helium Lines The figure below shows the. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. When hydrogen gas is placed. The emission spectra of various atoms. The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. Helium (he) strong lines of helium ( he ) intensity. The emission spectrum. Emission Spectra Helium Lines.
From hydrogenpotanezu.blogspot.com
Hydrogen Hydrogen Line Spectra Emission Spectra Helium Lines When hydrogen gas is placed. Helium (he) strong lines of helium ( he ) intensity. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. The figure below shows the. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to. Emission Spectra Helium Lines.
From commons.wikimedia.org
FileAtomic emission spectrum of helium.svg Wikimedia Commons Emission Spectra Helium Lines Helium (he) strong lines of helium ( he ) intensity. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. When hydrogen gas is placed. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of. Emission Spectra Helium Lines.
From www.chemhume.co.uk
Line emission spectra Emission Spectra Helium Lines The figure below shows the. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atoms of. Emission Spectra Helium Lines.
From www.dreamstime.com
The Spectrum Vector Diagram Stock Vector Illustration Emission Spectra Helium Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. A matching absorption spectrum occurs when light passes through a material and. Emission Spectra Helium Lines.
From www.researchgate.net
Optical emission spectra of helium arc with 20 vol hydrogen at Emission Spectra Helium Lines The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The figure below shows the. When hydrogen gas is placed. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. Helium (he) strong lines of helium (. Emission Spectra Helium Lines.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Helium Lines An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than. Emission Spectra Helium Lines.
From heliumoseiha.blogspot.com
Helium Emission Spectrum Of Helium Emission Spectra Helium Lines Helium (he) strong lines of helium ( he ) intensity. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectra of various atoms. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms.. Emission Spectra Helium Lines.
From www.chegg.com
Solved Refer to the atomic spectra of helium and krypton. Emission Spectra Helium Lines The figure below shows the. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectra of various atoms. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. The emission spectrum (or line. Emission Spectra Helium Lines.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Helium Lines Helium (he) strong lines of helium ( he ) intensity. The emission spectra of various atoms. The figure below shows the. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. A matching absorption spectrum occurs when light passes through a material and. Emission Spectra Helium Lines.
From fineartamerica.com
Emission Spectrum Of Helium Photograph by Dept. Of Physics, Imperial Emission Spectra Helium Lines Helium (he) strong lines of helium ( he ) intensity. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. A matching absorption spectrum occurs when light passes through a material and is absorbed by its atoms. When hydrogen gas is placed. An atomic. Emission Spectra Helium Lines.
From pixels.com
Helium Emission And Absorption Spectra Photograph by Carlos Clarivan Emission Spectra Helium Lines The 12 lines of the visible helium spectrum correspond to wavelengths of 388.8, 447.1,. The emission spectra of various atoms. When hydrogen gas is placed. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all. The emission spectrum (or line spectrum) of a chemical element is the unique pattern. Emission Spectra Helium Lines.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Emission Spectra Helium Lines Helium (he) strong lines of helium ( he ) intensity. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The figure below shows the. When hydrogen gas is placed. A matching absorption spectrum occurs when light passes through a material and is absorbed. Emission Spectra Helium Lines.