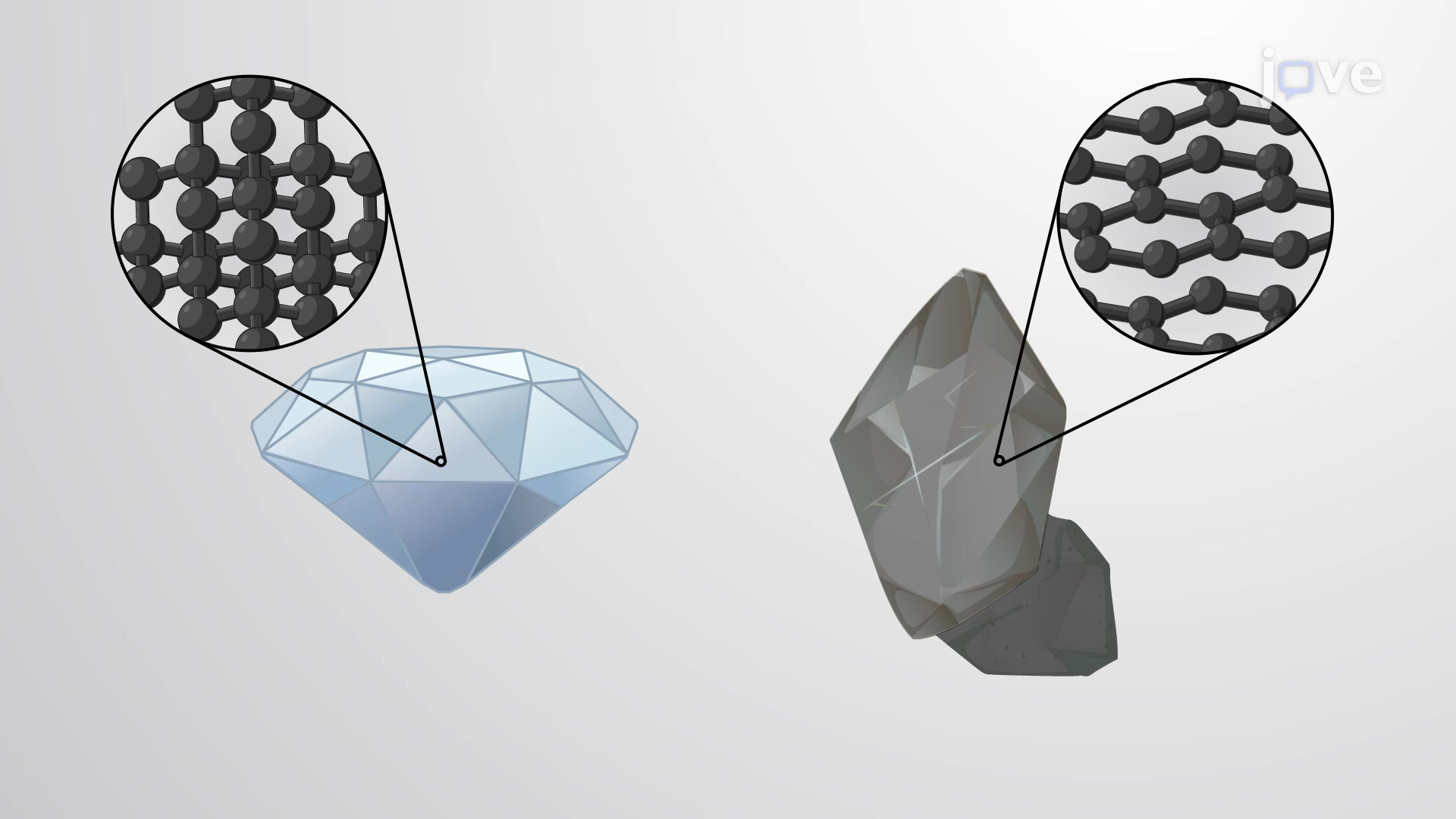
Graphite Network Solid . Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Examples of network solids include diamond with a continuous network of carbon. This page relates the structures of covalent network solids to the. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Each layer, however, is an endless bonded. Effectively the whole unit is. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. In a network solid there are no individual molecules and the entire crystal is the molecule. Network covalent structures are also called giant covalent structures or covalent network solids. For example, graphite is also a conductor of electricity along its. Other properties also depend on the plane of the crystal in network solids.
from app.jove.com
Each layer, however, is an endless bonded. This page relates the structures of covalent network solids to the. Other properties also depend on the plane of the crystal in network solids. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Network covalent structures are also called giant covalent structures or covalent network solids. In a network solid there are no individual molecules and the entire crystal is the molecule. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Effectively the whole unit is. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). For example, graphite is also a conductor of electricity along its.
Network Covalent Solids Diamond, Graphite and Quartz Concept
Graphite Network Solid In a network solid there are no individual molecules and the entire crystal is the molecule. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Effectively the whole unit is. In a network solid there are no individual molecules and the entire crystal is the molecule. For example, graphite is also a conductor of electricity along its. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. This page relates the structures of covalent network solids to the. Each layer, however, is an endless bonded. Other properties also depend on the plane of the crystal in network solids. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Examples of network solids include diamond with a continuous network of carbon. Network covalent structures are also called giant covalent structures or covalent network solids.
From www.coursehero.com
Covalent Crystals Introduction to Chemistry Course Hero Graphite Network Solid Effectively the whole unit is. Examples of network solids include diamond with a continuous network of carbon. Each layer, however, is an endless bonded. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Covalent network solids are giant covalent substances like diamond, graphite and. Graphite Network Solid.
From www.makethebrainhappy.com
MakeTheBrainHappy Covalent Network Solids Graphite Network Solid Other properties also depend on the plane of the crystal in network solids. Network covalent structures are also called giant covalent structures or covalent network solids. This page relates the structures of covalent network solids to the. For example, graphite is also a conductor of electricity along its. Examples of network solids include diamond with a continuous network of carbon.. Graphite Network Solid.
From www.youtube.com
Covalent Network Solids YouTube Graphite Network Solid Network covalent structures are also called giant covalent structures or covalent network solids. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Examples of network solids include diamond with a continuous network of carbon. In. Graphite Network Solid.
From www.slideserve.com
PPT Covalent Network Solids PowerPoint Presentation, free download Graphite Network Solid Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. This page relates the structures of covalent network solids to the. Other properties also depend on the plane of the crystal in network solids. In a network solid there are no individual molecules and the entire crystal is the molecule. A. Graphite Network Solid.
From www.youtube.com
Covalent network solids Intermolecular forces and properties AP Graphite Network Solid In a network solid there are no individual molecules and the entire crystal is the molecule. Effectively the whole unit is. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Examples of network solids include diamond with a continuous network of carbon. Covalent network. Graphite Network Solid.
From chem.libretexts.org
12.6 Network Covalent Atomic Solids Carbon and Silicates Chemistry Graphite Network Solid Each layer, however, is an endless bonded. Effectively the whole unit is. Examples of network solids include diamond with a continuous network of carbon. This page relates the structures of covalent network solids to the. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Covalent network solids are giant covalent substances like diamond, graphite. Graphite Network Solid.
From www.makethebrainhappy.com
MakeTheBrainHappy Covalent Network Solids Graphite Network Solid Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Network covalent structures are also called giant covalent structures or covalent network solids. Examples of network solids include diamond with a continuous network of carbon. This page relates the structures of covalent network solids to the. A giant molecular structure, or network solid, has a. Graphite Network Solid.
From www.youtube.com
Solids. YouTube Graphite Network Solid A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. This page relates the structures of covalent network solids to the. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Network covalent structures are also called giant covalent structures. Graphite Network Solid.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Graphite Network Solid Effectively the whole unit is. Network covalent structures are also called giant covalent structures or covalent network solids. Examples of network solids include diamond with a continuous network of carbon. Each layer, however, is an endless bonded. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong. Graphite Network Solid.
From www.askiitians.com
Classification of Crystalline Solids Study Material for IIT JEE Graphite Network Solid For example, graphite is also a conductor of electricity along its. This page relates the structures of covalent network solids to the. Effectively the whole unit is. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Each layer, however, is an endless bonded. Examples. Graphite Network Solid.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID355773 Graphite Network Solid Examples of network solids include diamond with a continuous network of carbon. Network covalent structures are also called giant covalent structures or covalent network solids. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). A. Graphite Network Solid.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk Graphite Network Solid Network covalent structures are also called giant covalent structures or covalent network solids. Effectively the whole unit is. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong. Graphite Network Solid.
From app.jove.com
Network Covalent Solids Diamond, Graphite and Quartz Concept Graphite Network Solid Other properties also depend on the plane of the crystal in network solids. This page relates the structures of covalent network solids to the. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Each layer, however, is an endless. Graphite Network Solid.
From www.nagwa.com
Lesson Video Network Covalent Structures Nagwa Graphite Network Solid Effectively the whole unit is. Network covalent structures are also called giant covalent structures or covalent network solids. In a network solid there are no individual molecules and the entire crystal is the molecule. Other properties also depend on the plane of the crystal in network solids. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide. Graphite Network Solid.
From theconversation.com
Harder than diamond, stronger than steel, super conductor graphene Graphite Network Solid Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Network covalent structures are also called giant covalent structures or covalent network solids. This page relates the structures of covalent network solids to the. Other properties also depend on the plane of the crystal in network solids. For example, graphite is. Graphite Network Solid.
From www.slideserve.com
PPT SOLID STATES PowerPoint Presentation, free download ID6018703 Graphite Network Solid Effectively the whole unit is. Other properties also depend on the plane of the crystal in network solids. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Each layer, however, is an endless bonded. Network covalent structures are also called giant covalent structures or. Graphite Network Solid.
From www.wou.edu
CH105 Chapter 4 The Shape and Characteristics of Compounds Chemistry Graphite Network Solid Other properties also depend on the plane of the crystal in network solids. Effectively the whole unit is. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. For example, graphite is also a conductor of electricity along its. This page relates the structures of. Graphite Network Solid.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free Graphite Network Solid Each layer, however, is an endless bonded. Network covalent structures are also called giant covalent structures or covalent network solids. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds.. Graphite Network Solid.
From www.chegg.com
Solved Graphite is a/an nonbonding atomic solid molecular Graphite Network Solid Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Network covalent structures are also called giant covalent structures or covalent network solids. In a network solid there are no individual molecules and the entire crystal. Graphite Network Solid.
From www.slideserve.com
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation Graphite Network Solid Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Effectively the whole unit is. Examples of network solids include diamond with a continuous network of carbon. Covalent network solids. Graphite Network Solid.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Graphite Network Solid In a network solid there are no individual molecules and the entire crystal is the molecule. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Network covalent structures are also called giant covalent structures or covalent network solids. Each layer, however, is an endless bonded. For example, graphite is also a conductor of electricity. Graphite Network Solid.
From www.slideserve.com
PPT Recap Bonding PowerPoint Presentation, free download ID2191626 Graphite Network Solid For example, graphite is also a conductor of electricity along its. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Network covalent structures are also called giant covalent structures or covalent network solids. Each layer, however, is an endless bonded. This page relates the structures of covalent network solids to the. Graphite may also. Graphite Network Solid.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID4500701 Graphite Network Solid Other properties also depend on the plane of the crystal in network solids. This page relates the structures of covalent network solids to the. Each layer, however, is an endless bonded. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Examples of network solids include diamond with a continuous network of carbon. Effectively the. Graphite Network Solid.
From chem.libretexts.org
12.4 The Fundamental Types of Crystalline Solids Chemistry LibreTexts Graphite Network Solid Effectively the whole unit is. Other properties also depend on the plane of the crystal in network solids. Network covalent structures are also called giant covalent structures or covalent network solids. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. This page relates the. Graphite Network Solid.
From www.slideserve.com
PPT Unit 5 Bonding PowerPoint Presentation, free download ID4396191 Graphite Network Solid Effectively the whole unit is. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Network covalent structures are also called giant covalent structures or covalent. Graphite Network Solid.
From courses.lumenlearning.com
Covalent Crystals Introduction to Chemistry Graphite Network Solid Effectively the whole unit is. Network covalent structures are also called giant covalent structures or covalent network solids. In a network solid there are no individual molecules and the entire crystal is the molecule. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Each layer, however, is an endless bonded.. Graphite Network Solid.
From www.youtube.com
ib6.8 covalent network solids YouTube Graphite Network Solid Each layer, however, is an endless bonded. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. For example, graphite is also a conductor of electricity along its. This page relates the structures of covalent network solids to the. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite Network Solid.
From www.slideserve.com
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation Graphite Network Solid A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. Each layer, however, is an endless bonded. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Network covalent structures are also called giant covalent structures or covalent network solids.. Graphite Network Solid.
From superiorgraphite.com
About Graphite Graphite Products Graphite Network Solid Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of which are bonded into position using strong covalent bonds. For example, graphite is also a conductor of electricity along its. In a network solid there are no individual molecules. Graphite Network Solid.
From www.sliderbase.com
Network Atomic Solids Graphite Network Solid In a network solid there are no individual molecules and the entire crystal is the molecule. Other properties also depend on the plane of the crystal in network solids. Each layer, however, is an endless bonded. Effectively the whole unit is. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). A giant molecular structure,. Graphite Network Solid.
From www.nisenet.org
Scientific Image Graphite models NISE Network Graphite Network Solid Examples of network solids include diamond with a continuous network of carbon. Each layer, however, is an endless bonded. Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Network covalent structures are also called giant. Graphite Network Solid.
From www.slideserve.com
PPT Recap Bonding PowerPoint Presentation, free download ID2191626 Graphite Network Solid Effectively the whole unit is. In a network solid there are no individual molecules and the entire crystal is the molecule. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Covalent network solids are giant. Graphite Network Solid.
From substech.com
Graphite as solid lubricant [SubsTech] Graphite Network Solid Graphite may also be regarded as a network solid, even though there is no bonding in the z direction. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). For example, graphite is also a conductor of electricity along its. Effectively the whole unit is. Network covalent structures are also called giant covalent structures or. Graphite Network Solid.
From www.youtube.com
Covalent Network Solids YouTube Graphite Network Solid Network covalent structures are also called giant covalent structures or covalent network solids. In a network solid there are no individual molecules and the entire crystal is the molecule. This page relates the structures of covalent network solids to the. Other properties also depend on the plane of the crystal in network solids. Effectively the whole unit is. Covalent network. Graphite Network Solid.
From www.youtube.com
Ionic Solids, Molecular Solids, Metallic Solids, Network Covalent Graphite Network Solid Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). This page relates the structures of covalent network solids to the. Other properties also depend on the plane of the crystal in network solids. Effectively the whole unit is. A giant molecular structure, or network solid, has a virtually infinite arrangement of atoms, all of. Graphite Network Solid.