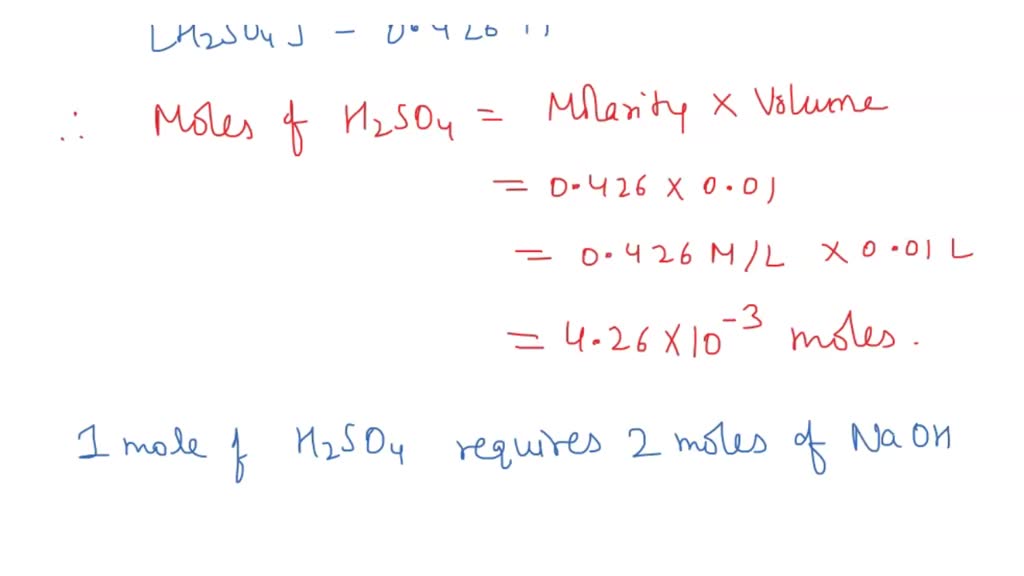Calculation Titration Sulphuric Acid Sodium Hydroxide . The sulphuric acid has an unknown concentration. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Then you will fill a burette with sodium. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of.
from www.numerade.com
In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. Then you will fill a burette with sodium. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. The sulphuric acid has an unknown concentration.
SOLVED Consider the titration of sulfuric acid with sodium hydroxide
Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Then you will fill a burette with sodium. In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The sulphuric acid has an unknown concentration. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used.
From www.slideserve.com
PPT Transition Metals (Cr, Mn , Fe, Co, Ni and Cu) PowerPoint Calculation Titration Sulphuric Acid Sodium Hydroxide The sulphuric acid has an unknown concentration. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. This is the volume of acid that. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From ar.inspiredpencil.com
Sulfuric Acid And Sodium Hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.coursehero.com
[Solved] For the titration of sulfuric acid (H2SO4) with sodium hyd Calculation Titration Sulphuric Acid Sodium Hydroxide This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. That means number. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.myxxgirl.com
Sulphuric Acid Sodium Hydroxide My XXX Hot Girl Calculation Titration Sulphuric Acid Sodium Hydroxide This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Then you will fill a burette with sodium. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. The example below demonstrates the technique to solve a. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From slideplayer.com
TITRATIONS LESSON OBJECTIVE At the end of the lesson you should be able Calculation Titration Sulphuric Acid Sodium Hydroxide In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. The example below demonstrates the technique to solve. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.numerade.com
SOLVED 12 mL of 0.25 N sulphuric acid is neutralised with 15 mL of Calculation Titration Sulphuric Acid Sodium Hydroxide The sulphuric acid has an unknown concentration. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.youtube.com
Acid Base Titration Sulfuric acid and Sodium hydroxide YouTube Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Then you will fill a burette with sodium. In this experiment, you will use a pipette to measure some sulphuric. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.numerade.com
SOLVED Concentration Calculations Sodium Hydroxide Calculate the Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. The sulphuric acid has an unknown concentration. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.youtube.com
Sodium Hydroxide + Sulfuric Acid Acid Base Neutralization Reaction Calculation Titration Sulphuric Acid Sodium Hydroxide In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration.. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From gbu-presnenskij.ru
Titration Of Sulfuric Acid And Sodium Hydroxide Science, 45 OFF Calculation Titration Sulphuric Acid Sodium Hydroxide In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. The. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From syatillakmk.blogspot.com
SimplyChemistry TITRATION OF SULFURIC ACID WITH SODIUM HYDROXIDE Calculation Titration Sulphuric Acid Sodium Hydroxide Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. The. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.toppr.com
12 mL of 0.25 N sulphuric acid is neutralised with 15 mL of sodium Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. Then you will fill a burette with sodium. That means number of moles of sulfuric. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From ar.inspiredpencil.com
Sulfuric Acid And Sodium Hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. The sulphuric acid has an unknown concentration. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. That means number of moles of sulfuric acid is half that of number of moles of. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.numerade.com
SOLVED Q3 In a titration of sulfuric acid against sodium hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From brainly.com
A solution of sulfuric acid is titrated with a solution of sodium Calculation Titration Sulphuric Acid Sodium Hydroxide In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. The sulphuric acid has an unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. The example below demonstrates the technique to solve a titration problem for. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From dokumen.tips
(DOCX) Titration of Sulfuric Acid With Sodium Hydroxide DOKUMEN.TIPS Calculation Titration Sulphuric Acid Sodium Hydroxide Then you will fill a burette with sodium. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. The sulphuric acid has an unknown concentration. In my latest chem lab the objective was to create a primary standard of $\ce. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.studypool.com
SOLUTION Introduction to titration determination of the molarity and Calculation Titration Sulphuric Acid Sodium Hydroxide In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. Then you will fill a burette with sodium. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. In. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.youtube.com
Sodium Hydroxide and Sulfuric Acid yields Sodium sulfate and Water Calculation Titration Sulphuric Acid Sodium Hydroxide The sulphuric acid has an unknown concentration. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From ar.inspiredpencil.com
Sulfuric Acid And Sodium Hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Then you will fill a burette with. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.numerade.com
SOLVED Consider the titration of sulfuric acid with sodium hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In my latest chem lab the objective was to create a primary standard of. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.numerade.com
SOLVED ACIDE BASE TITRATIONS DATA COLLECTION Table Sodium hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. Then you will fill a burette with sodium. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. This is the volume of acid that. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.scribd.com
Diprotic Acid Titration Calculation Worked Example Sulphuric Acid and Calculation Titration Sulphuric Acid Sodium Hydroxide In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Then you will fill a burette with sodium. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. The sulphuric acid has an unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.numerade.com
SOLVED For the acidbase reaction (called titration) of sulfuric acid Calculation Titration Sulphuric Acid Sodium Hydroxide In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The sulphuric acid has an unknown concentration. Sulfuric acid reacts with sodium hydroxide on the. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From gbu-presnenskij.ru
Titration Of Sulfuric Acid And Sodium Hydroxide Science, 45 OFF Calculation Titration Sulphuric Acid Sodium Hydroxide Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. In my latest chem lab the objective was to create a primary standard of. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From ar.inspiredpencil.com
Sulfuric Acid And Sodium Hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The sulphuric acid has an unknown concentration. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. This is the volume of acid that exactly reacts with the sodium hydroxide solution. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From ar.inspiredpencil.com
Sulfuric Acid And Sodium Hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide The sulphuric acid has an unknown concentration. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide.. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.numerade.com
SOLVED In the titration of sulfuric acid against sodium hydroxide, 32. Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From norah-chapter.blogspot.com
Sodium Bicarbonate And Sulfuric Acid Equation 20+ Pages Explanation Doc Calculation Titration Sulphuric Acid Sodium Hydroxide Then you will fill a burette with sodium. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The sulphuric acid. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From ar.inspiredpencil.com
Sulfuric Acid And Sodium Hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. Then you will. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.gauthmath.com
Solved QUESTION 3 To determine the enthalpy change of neutralization Calculation Titration Sulphuric Acid Sodium Hydroxide Then you will fill a burette with sodium. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. In a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute solution of. The sulphuric acid has an unknown concentration. In. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.numerade.com
SOLVED what happens when sodium hydroxide reacts with sulphuric acid Calculation Titration Sulphuric Acid Sodium Hydroxide That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. The sulphuric acid has an unknown concentration. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis.. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Calculation Titration Sulphuric Acid Sodium Hydroxide This is the volume of acid that exactly reacts with the sodium hydroxide solution of unknown concentration. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. That means number of moles of sulfuric. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.youtube.com
Titration calculation for sodium hydroxide and sulfuric acid YouTube Calculation Titration Sulphuric Acid Sodium Hydroxide In my latest chem lab the objective was to create a primary standard of $\ce {naoh}$ and use it to determine the concentration of. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. The sulphuric acid has an unknown concentration. The example below demonstrates the technique to solve a titration problem for a. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.studypool.com
SOLUTION Acid base titration of sulphuric acid and sodium hydroxide Calculation Titration Sulphuric Acid Sodium Hydroxide In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Then you will fill a burette with sodium. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. This is the volume of acid. Calculation Titration Sulphuric Acid Sodium Hydroxide.
From www.bartleby.com
Answered Q3 In a titration of sulfuric acid… bartleby Calculation Titration Sulphuric Acid Sodium Hydroxide The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. The example below demonstrates the technique to solve a titration problem. Calculation Titration Sulphuric Acid Sodium Hydroxide.