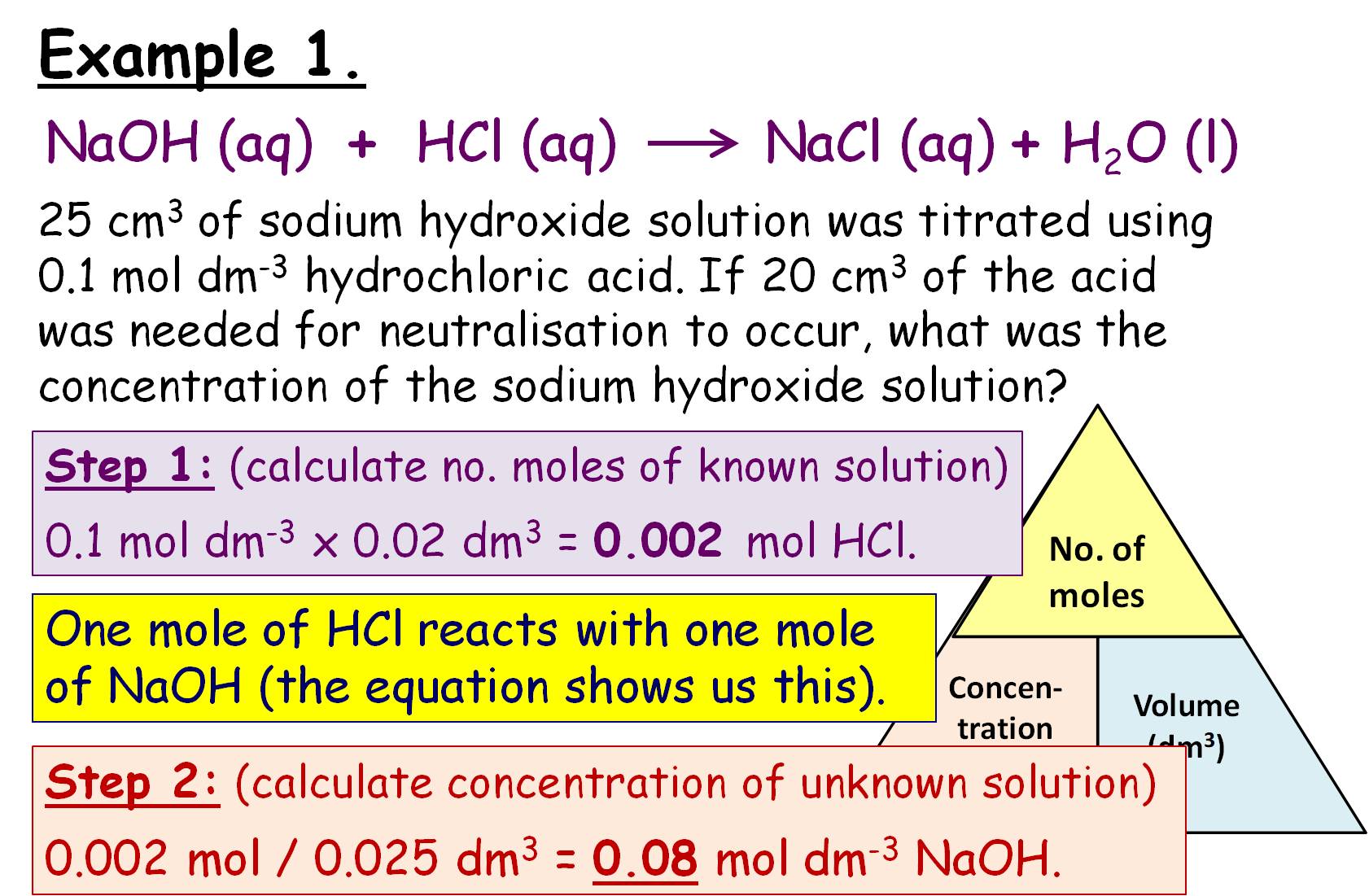
Titration Calculations Worksheet Answers . Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Answers to the titrations practice worksheet. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. In a titration, we measure the volume of an acid (or. Titrations are a very accurate way of measuring the concentration of acids and alkalis. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid.
from www.vrogue.co
For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Answers to the titrations practice worksheet. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. In a titration, we measure the volume of an acid (or. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid.
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co
Titration Calculations Worksheet Answers 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. Answers to the titrations practice worksheet. In a titration, we measure the volume of an acid (or. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base.
From www.vrogue.co
Titration Calculations Worksheet Professor Feito Twin vrogue.co Titration Calculations Worksheet Answers 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my. Titration Calculations Worksheet Answers.
From www.vrogue.co
Acid Base Titration Worksheet Calculations Including vrogue.co Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Titrations are a very accurate way of measuring the concentration of acids and alkalis. In a titration, we measure the volume of an acid (or. For questions 1 and 2, the units for your final answer. Titration Calculations Worksheet Answers.
From worksheetzonegibson55.z21.web.core.windows.net
Titrations Practice Worksheet Answers Titration Calculations Worksheet Answers Titrations are a very accurate way of measuring the concentration of acids and alkalis. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
Solved Titration calculations worksheet 1. You measured out Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. For questions 1 and 2, the units for your final answer should be “m”, or “molar”,. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
Free titrations practice worksheet answers, Download Free titrations Titration Calculations Worksheet Answers 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Titrations are a very accurate way of measuring the concentration of acids and alkalis. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed. Titration Calculations Worksheet Answers.
From www.vrogue.co
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co Titration Calculations Worksheet Answers Titrations are a very accurate way of measuring the concentration of acids and alkalis. In a titration, we measure the volume of an acid (or. Answers to the titrations practice worksheet. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. For questions 1 and 2, the units for your. Titration Calculations Worksheet Answers.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Titration Calculations Worksheet Answers For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Revision notes on titration calculations for the aqa. Titration Calculations Worksheet Answers.
From studylib.net
Titration Practice Worksheet Titration Calculations Worksheet Answers 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying. Titration Calculations Worksheet Answers.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Titration Calculations Worksheet Answers Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a. Titration Calculations Worksheet Answers.
From www.vrogue.co
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. In a. Titration Calculations Worksheet Answers.
From worksheetzonegibson55.z21.web.core.windows.net
Titration Worksheet Answer Key Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Answers to the titrations practice worksheet. In a titration, we measure the volume of an acid (or. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
How to Perform a Titration with Equivalent Weak Acid & Strong Base Titration Calculations Worksheet Answers 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. In a titration, we measure the volume of an acid (or. Answers to the titrations practice worksheet. Titrations are a very. Titration Calculations Worksheet Answers.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 ans 93ghs Studypool Titration Calculations Worksheet Answers 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Titrations are a very accurate way of measuring the concentration of acids and alkalis. In a titration, we measure the volume of an acid (or. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to. Titration Calculations Worksheet Answers.
From www.edplace.com
Understand Advanced Titration Calculations Worksheet EdPlace Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. In a titration, we measure the volume of an acid (or. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. For questions 1 and 2,. Titration Calculations Worksheet Answers.
From dorkiestenews.blogspot.com
Acid Base Titration Worksheet Answers Acid Base Titration Worksheet Titration Calculations Worksheet Answers Titrations are a very accurate way of measuring the concentration of acids and alkalis. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. 25.0 cm3. Titration Calculations Worksheet Answers.
From www.tes.com
Titrations Home Learning Worksheet GCSE Teaching Resources Titration Calculations Worksheet Answers Answers to the titrations practice worksheet. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. In a titration, we measure the volume of an acid (or. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
Solved Titrations Practice WorksheetFxtra practice Find the Titration Calculations Worksheet Answers In a titration, we measure the volume of an acid (or. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Answers. Titration Calculations Worksheet Answers.
From mungfali.com
Titration Worksheet Titration Calculations Worksheet Answers Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Answers. Titration Calculations Worksheet Answers.
From www.vrogue.co
Acid Base Titration Worksheet Answers W336 Titrations vrogue.co Titration Calculations Worksheet Answers For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Answers to the titrations practice worksheet. Titrations are a very accurate way of measuring the concentration of. Titration Calculations Worksheet Answers.
From studylib.net
acid base titration worksheet answer key Titration Calculations Worksheet Answers 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. In a titration, we measure the volume of an acid (or. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples. Titration Calculations Worksheet Answers.
From www.vrogue.co
Titration Calculations And Questions Worksheet Teachi vrogue.co Titration Calculations Worksheet Answers For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. In a titration, we measure the volume of an acid (or. Titrations are a very accurate way of measuring the concentration of acids and alkalis. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
How to Perform a Titration with Equivalent Weak Acid & Strong Base Titration Calculations Worksheet Answers Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. 25.0 cm3. Titration Calculations Worksheet Answers.
From www.studocu.com
Titration Calculations TITRATION CALCULATIONS WORKSHEET A 50 ml Titration Calculations Worksheet Answers Titrations are a very accurate way of measuring the concentration of acids and alkalis. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Answers. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
Acid Base Titrations Worksheet Worksheets Library Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Revision notes on titration calculations for the aqa. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
Titration Practice Acid Base Reaction Worksheet with Answer Key Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Revision notes on titration calculations for the. Titration Calculations Worksheet Answers.
From www.studocu.com
Examples of back titration w answers 2008 MCHE223 Studocu Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. In a titration, we measure the volume of an acid (or. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. Answers to the titrations practice worksheet. For questions. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
Titration Problems worksheet Live Worksheets Worksheets Library Titration Calculations Worksheet Answers 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration,. Titration Calculations Worksheet Answers.
From www.tes.com
Titrations Home Learning Worksheet GCSE Teaching Resources Titration Calculations Worksheet Answers 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration,. Titration Calculations Worksheet Answers.
From studylib.net
Titration Worksheet Titration Calculations Worksheet Answers Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Answers to the titrations practice worksheet. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6. Titration Calculations Worksheet Answers.
From quizzlistjonas.z19.web.core.windows.net
Titration Worksheet Answer Key Titration Calculations Worksheet Answers 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
Weak Acid Base Titration worksheet Weak Acid Base Titration Titration Calculations Worksheet Answers Ch3cooh(aq) + naoh(aq) → ch3coona (aq) + h2o(l) in each titration, a student placed 25.0 cm3 samples of the vinegar in a conical flask. Answers to the titrations practice worksheet. In a titration, we measure the volume of an acid (or. Titrations are a very accurate way of measuring the concentration of acids and alkalis. Revision notes on titration calculations. Titration Calculations Worksheet Answers.
From www.scribd.com
titration_answer_keys PDF Titration Calculations Worksheet Answers For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Revision notes on titration calculations for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Answers to the titrations practice worksheet. Titrations are a very accurate way. Titration Calculations Worksheet Answers.
From wks.udlvirtual.edu.pe
Acid Base Titration Practice Worksheet Worksheets Printable Free Titration Calculations Worksheet Answers Answers to the titrations practice worksheet. 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. In a titration, we measure the volume of an acid (or. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. Revision notes on titration calculations for the aqa gcse chemistry syllabus,. Titration Calculations Worksheet Answers.
From worksheets.clipart-library.com
Free Printable Acids and Bases Titration Worksheets Worksheets Library Titration Calculations Worksheet Answers 25.0 cm3 of 0.150 mol/dm3 sodium hydroxide reacted with 30.3 cm3 of a solution of ethanoic acid. In a titration, we measure the volume of an acid (or. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. Titrations are a very. Titration Calculations Worksheet Answers.
From www.vrogue.co
Titration Calculations Worksheet Gcse Chemistry Beyon vrogue.co Titration Calculations Worksheet Answers Titrations are a very accurate way of measuring the concentration of acids and alkalis. 1 25.0 cm3 of 0.200 mol/dm3 sulfuric acid neutralises 18.6 cm3 of barium hydroxide solution. For questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the acid or base. 25.0 cm3 of. Titration Calculations Worksheet Answers.