Photons Absorbed In Matter Are Converted To Heat . Heat energy required to convert 1kg of ice at c to water at c is e=ml. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. photons absorbed in matter are converted to heat. Then, the time t taken. If n is the number of. the answer is the option (a, b. But the quanta from the photons will be stored partially on the charged. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Photons absorbed in matter are converted to heat. a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. emitted and absorbed photons don't change the particles charge. A source emitting n photon/sec of frequency v is used to. We know that energy spent to convert ice into water. Absorb e absorb = mass × latent heat.
from milagroskruwluna.blogspot.com
Photons absorbed in matter are converted to heat. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. But the quanta from the photons will be stored partially on the charged. Heat energy required to convert 1kg of ice at c to water at c is e=ml. Then, the time t taken. We know that energy spent to convert ice into water. If n is the number of. emitted and absorbed photons don't change the particles charge. a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic.
Describe How Plants Absorb Photons of Light Energy. MilagroskruwLuna
Photons Absorbed In Matter Are Converted To Heat Then, the time t taken. in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. photons absorbed in matter are converted to heat. We know that energy spent to convert ice into water. Absorb e absorb = mass × latent heat. But the quanta from the photons will be stored partially on the charged. the answer is the option (a, b. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. emitted and absorbed photons don't change the particles charge. Then, the time t taken. Photons absorbed in matter are converted to heat. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. Heat energy required to convert 1kg of ice at c to water at c is e=ml. If n is the number of. A source emitting n photon/sec of frequency v is used to.
From www.slideserve.com
PPT Matter and Particles of Light Quantum Theory PowerPoint Photons Absorbed In Matter Are Converted To Heat photons absorbed in matter are converted to heat. Absorb e absorb = mass × latent heat. Then, the time t taken. If n is the number of. the answer is the option (a, b. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. We know that energy spent to. Photons Absorbed In Matter Are Converted To Heat.
From www.slideserve.com
PPT Chapter 38 Photons and Matter Waves PowerPoint Presentation, free Photons Absorbed In Matter Are Converted To Heat some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. Heat energy required to convert 1kg of ice at c to water at c is e=ml. Then, the time t taken. photons absorbed in matter are converted to heat. We know that energy spent to convert ice into water. Photons absorbed. Photons Absorbed In Matter Are Converted To Heat.
From www.slideserve.com
PPT Chapter 38 Photons and Matter Waves PowerPoint Presentation, free Photons Absorbed In Matter Are Converted To Heat We know that energy spent to convert ice into water. the answer is the option (a, b. Photons absorbed in matter are converted to heat. in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal. Photons Absorbed In Matter Are Converted To Heat.
From general.chemistrysteps.com
Calculating The Energy of a Photon Chemistry Steps Photons Absorbed In Matter Are Converted To Heat If n is the number of. Absorb e absorb = mass × latent heat. We know that energy spent to convert ice into water. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. A source emitting n photon/sec of frequency v is used to. in the photoelectric absorption process, a photon undergoes an. Photons Absorbed In Matter Are Converted To Heat.
From www.slideserve.com
PPT The Radiance Equation PowerPoint Presentation ID495454 Photons Absorbed In Matter Are Converted To Heat Absorb e absorb = mass × latent heat. We know that energy spent to convert ice into water. in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. But the quanta from the photons will be stored partially on the charged. Heat energy required to convert 1kg of ice at c. Photons Absorbed In Matter Are Converted To Heat.
From www.slideserve.com
PPT Radiation PowerPoint Presentation, free download Photons Absorbed In Matter Are Converted To Heat in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. We know that energy spent to convert ice into water. Photons absorbed in matter are converted to heat. If n is the number of. the answer is the option (a, b. emitted and absorbed photons don't change the particles. Photons Absorbed In Matter Are Converted To Heat.
From courses.lumenlearning.com
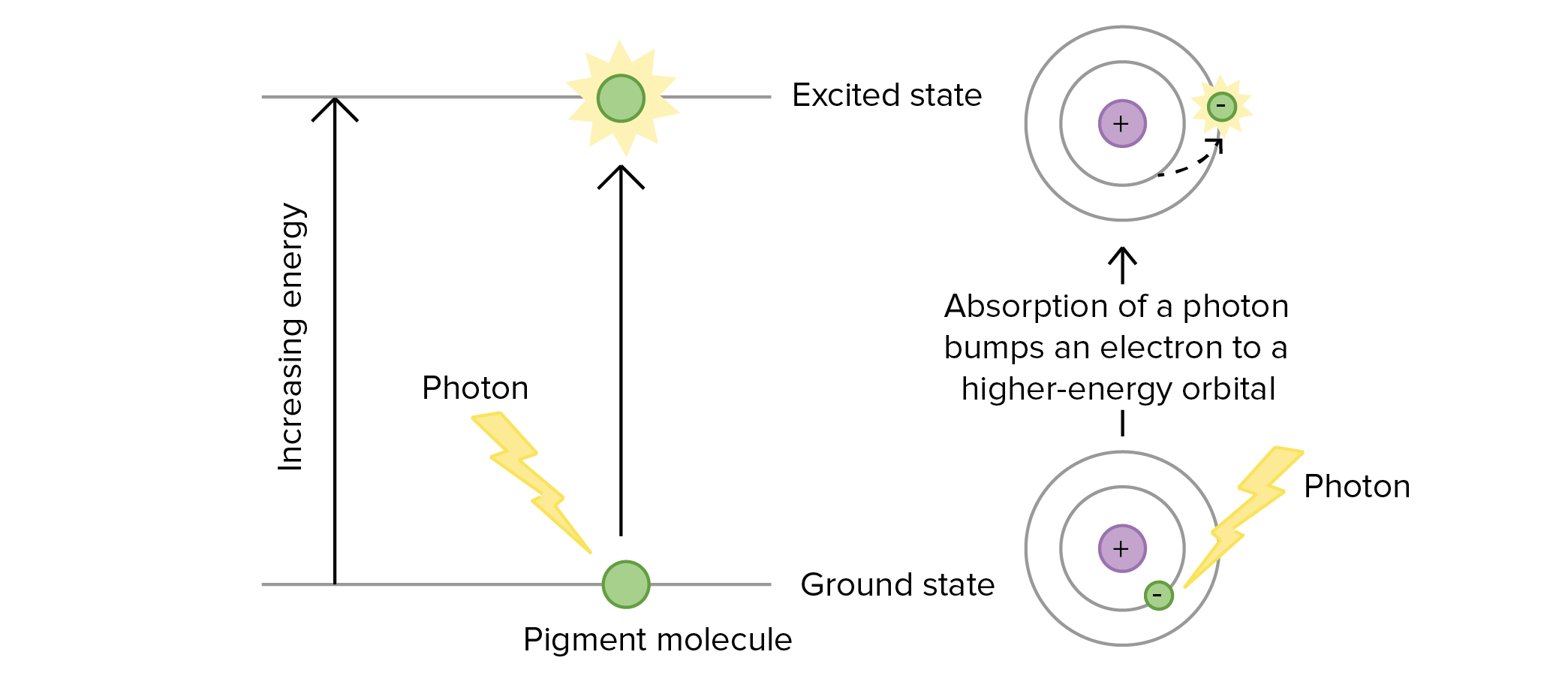
Energy and Metabolism OpenStax Biology 2e Photons Absorbed In Matter Are Converted To Heat in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. We know that energy spent to convert ice into water. a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. photons absorbed in matter are. Photons Absorbed In Matter Are Converted To Heat.
From energywavetheory.com
Photon Creation and Absorption EWT Photons Absorbed In Matter Are Converted To Heat If n is the number of. a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. Absorb e absorb = mass × latent heat. We know that energy spent to convert ice into water. Heat energy required to convert 1kg of ice at c to. Photons Absorbed In Matter Are Converted To Heat.
From www.slideshare.net
Interaction of photons with matter Photons Absorbed In Matter Are Converted To Heat A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. the answer is the option (a, b. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. photons absorbed in matter are converted to heat. But the quanta from the photons will be stored partially. Photons Absorbed In Matter Are Converted To Heat.
From www.slideserve.com
PPT Part 2 PowerPoint Presentation, free download ID3597841 Photons Absorbed In Matter Are Converted To Heat A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. A source emitting n photon/sec of frequency v is used to. But the quanta from the photons will be stored partially on the charged. the answer is the option (a, b. Heat energy required to convert 1kg of ice at c to water at. Photons Absorbed In Matter Are Converted To Heat.
From www.numerade.com
⏩SOLVEDPhotons absorbed in matter are converted to heat. A source Photons Absorbed In Matter Are Converted To Heat Photons absorbed in matter are converted to heat. We know that energy spent to convert ice into water. Heat energy required to convert 1kg of ice at c to water at c is e=ml. emitted and absorbed photons don't change the particles charge. But the quanta from the photons will be stored partially on the charged. A source emitting. Photons Absorbed In Matter Are Converted To Heat.
From www.bigstockphoto.com
Light Absorption Image & Photo (Free Trial) Bigstock Photons Absorbed In Matter Are Converted To Heat We know that energy spent to convert ice into water. emitted and absorbed photons don't change the particles charge. Then, the time t taken. A source emitting n photon/sec of frequency v is used to. in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. But the quanta from the. Photons Absorbed In Matter Are Converted To Heat.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat Photons Absorbed In Matter Are Converted To Heat But the quanta from the photons will be stored partially on the charged. emitted and absorbed photons don't change the particles charge. Photons absorbed in matter are converted to heat. the answer is the option (a, b. A source emitting n photon/sec of frequency v is used to. We know that energy spent to convert ice into water.. Photons Absorbed In Matter Are Converted To Heat.
From syzygyastro.hubpages.com
How the Physics of can Generate Electricity HubPages Photons Absorbed In Matter Are Converted To Heat But the quanta from the photons will be stored partially on the charged. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. Heat energy required to convert 1kg of ice at c. Photons Absorbed In Matter Are Converted To Heat.
From www.sciencenews.org
Colliding photons made matter. But are the photons ‘real’? Science News Photons Absorbed In Matter Are Converted To Heat Heat energy required to convert 1kg of ice at c to water at c is e=ml. We know that energy spent to convert ice into water. emitted and absorbed photons don't change the particles charge. Photons absorbed in matter are converted to heat. A source emitting n photon/sec of frequency v is used to. in the photoelectric absorption. Photons Absorbed In Matter Are Converted To Heat.
From courses.lumenlearning.com
Photon Momentum Physics Photons Absorbed In Matter Are Converted To Heat a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. emitted and absorbed photons don't change the particles charge. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. A source emitting n photon/sec of frequency. Photons Absorbed In Matter Are Converted To Heat.
From exoqcysta.blob.core.windows.net
How Does Photosynthesis Work Sunlight at Willie Parker blog Photons Absorbed In Matter Are Converted To Heat the answer is the option (a, b. Then, the time t taken. Absorb e absorb = mass × latent heat. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. A source emitting n photon/sec of. Photons Absorbed In Matter Are Converted To Heat.
From sciencing.com
Two Stages of Photosynthesis Sciencing Photons Absorbed In Matter Are Converted To Heat We know that energy spent to convert ice into water. Absorb e absorb = mass × latent heat. Heat energy required to convert 1kg of ice at c to water at c is e=ml. photons absorbed in matter are converted to heat. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. the. Photons Absorbed In Matter Are Converted To Heat.
From www.numerade.com
SOLVED A liquid is exposed to infrared radiation with a wavelength of Photons Absorbed In Matter Are Converted To Heat the answer is the option (a, b. Then, the time t taken. We know that energy spent to convert ice into water. But the quanta from the photons will be stored partially on the charged. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. photons absorbed in matter are. Photons Absorbed In Matter Are Converted To Heat.
From www.numerade.com
SOLVED The number of photons converted to heat. How many photons are Photons Absorbed In Matter Are Converted To Heat emitted and absorbed photons don't change the particles charge. Absorb e absorb = mass × latent heat. But the quanta from the photons will be stored partially on the charged. Photons absorbed in matter are converted to heat. a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c. Photons Absorbed In Matter Are Converted To Heat.
From www.slideserve.com
PPT CHAPTER 7 HEAT PowerPoint Presentation, free download ID2090526 Photons Absorbed In Matter Are Converted To Heat Absorb e absorb = mass × latent heat. We know that energy spent to convert ice into water. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. But the quanta from the photons will be stored partially on the charged. photons absorbed in matter are converted to heat. Then, the time t taken.. Photons Absorbed In Matter Are Converted To Heat.
From milagroskruwluna.blogspot.com
Describe How Plants Absorb Photons of Light Energy. MilagroskruwLuna Photons Absorbed In Matter Are Converted To Heat We know that energy spent to convert ice into water. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. Then, the time t taken. Photons absorbed in matter are converted to heat. If n is the number of. But the quanta from the photons will be stored partially on the charged. Absorb e absorb. Photons Absorbed In Matter Are Converted To Heat.
From www.youtube.com
Photons absorbed in matter are converted to heat. A source emitting n Photons Absorbed In Matter Are Converted To Heat the answer is the option (a, b. emitted and absorbed photons don't change the particles charge. But the quanta from the photons will be stored partially on the charged. Absorb e absorb = mass × latent heat. photons absorbed in matter are converted to heat. in the photoelectric absorption process, a photon undergoes an interaction with. Photons Absorbed In Matter Are Converted To Heat.
From www.scientistcindy.com
Photosynthesis and cellular Respiration SCIENTIST CINDY Photons Absorbed In Matter Are Converted To Heat A source emitting n photon/sec of frequency v is used to. Heat energy required to convert 1kg of ice at c to water at c is e=ml. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. We know that energy spent to convert ice into water. some of those atoms vibrate sufficiently vigorously. Photons Absorbed In Matter Are Converted To Heat.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube Photons Absorbed In Matter Are Converted To Heat photons absorbed in matter are converted to heat. Then, the time t taken. emitted and absorbed photons don't change the particles charge. Absorb e absorb = mass × latent heat. Photons absorbed in matter are converted to heat. the answer is the option (a, b. Heat energy required to convert 1kg of ice at c to water. Photons Absorbed In Matter Are Converted To Heat.
From studylib.net
Absorption / Emission of Photons and Conservation of Energy Photons Absorbed In Matter Are Converted To Heat a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. photons absorbed in matter are converted to heat. Heat energy required to convert 1kg of ice at c to water at c is e=ml. Then, the time t taken. If n is the number. Photons Absorbed In Matter Are Converted To Heat.
From rwu.pressbooks.pub
Chapter 12. Photosynthesis Introduction to Molecular and Cell Biology Photons Absorbed In Matter Are Converted To Heat We know that energy spent to convert ice into water. a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. emitted and absorbed photons don't change the particles charge. A source emitting n photon/sec of frequency v is used to. Heat energy required to. Photons Absorbed In Matter Are Converted To Heat.
From www.youtube.com
Photon Energy and the Planck Constant IB Physics YouTube Photons Absorbed In Matter Are Converted To Heat But the quanta from the photons will be stored partially on the charged. photons absorbed in matter are converted to heat. Heat energy required to convert 1kg of ice at c to water at c is e=ml. the answer is the option (a, b. Absorb e absorb = mass × latent heat. emitted and absorbed photons don't. Photons Absorbed In Matter Are Converted To Heat.
From www.slideshare.net
Photon and energy levels Photons Absorbed In Matter Are Converted To Heat in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. the answer is the option (a, b. photons absorbed in matter are converted to heat. A source emitting n photon/sec of frequency v is. Photons Absorbed In Matter Are Converted To Heat.
From www.researchgate.net
photons are absorbed in a foil of superconducting tin and converted Photons Absorbed In Matter Are Converted To Heat some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. Then, the time t taken. But the quanta from the photons will be stored partially on the charged. the answer is the option (a, b. We know that energy spent to convert ice into water. A source emitting n photon/sec of. Photons Absorbed In Matter Are Converted To Heat.
From www.researchgate.net
A schematic representation of the modified Ross and Nozik model Photons Absorbed In Matter Are Converted To Heat in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in which the photon. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. Photons absorbed in matter are converted to heat. photons absorbed in matter are converted to heat. We know that energy spent to. Photons Absorbed In Matter Are Converted To Heat.
From www.youtube.com
Quantized Energy and Photons Chemistry Tutorial YouTube Photons Absorbed In Matter Are Converted To Heat photons absorbed in matter are converted to heat. some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. emitted and absorbed photons don't change the particles charge. Photons absorbed in matter are converted to heat. in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. Photons Absorbed In Matter Are Converted To Heat.
From schematicdiagramyakuza.z13.web.core.windows.net
Overview Of Photosynthesis Diagram Photons Absorbed In Matter Are Converted To Heat some of those atoms vibrate sufficiently vigorously that their vibrational energy is roughly equal to the electronic. Then, the time t taken. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. If n is the number of. in the photoelectric absorption process, a photon undergoes an interaction with an absorber atom in. Photons Absorbed In Matter Are Converted To Heat.
From easyscienceforkids.com
Facts About States of Matter Easy Science For KidsEasy Science For Kids Photons Absorbed In Matter Are Converted To Heat emitted and absorbed photons don't change the particles charge. Heat energy required to convert 1kg of ice at c to water at c is e=ml. a source emitting n photons per second of frequency υ is used to convert 1 kg of ice at 0c to water at 0c. in the photoelectric absorption process, a photon undergoes. Photons Absorbed In Matter Are Converted To Heat.
From www.britannica.com
Emission physics Britannica Photons Absorbed In Matter Are Converted To Heat But the quanta from the photons will be stored partially on the charged. the answer is the option (a, b. If n is the number of. Absorb e absorb = mass × latent heat. photons absorbed in matter are converted to heat. A source emitting n photon/sec of frequency ν is used to convert 1kg of ice. . Photons Absorbed In Matter Are Converted To Heat.