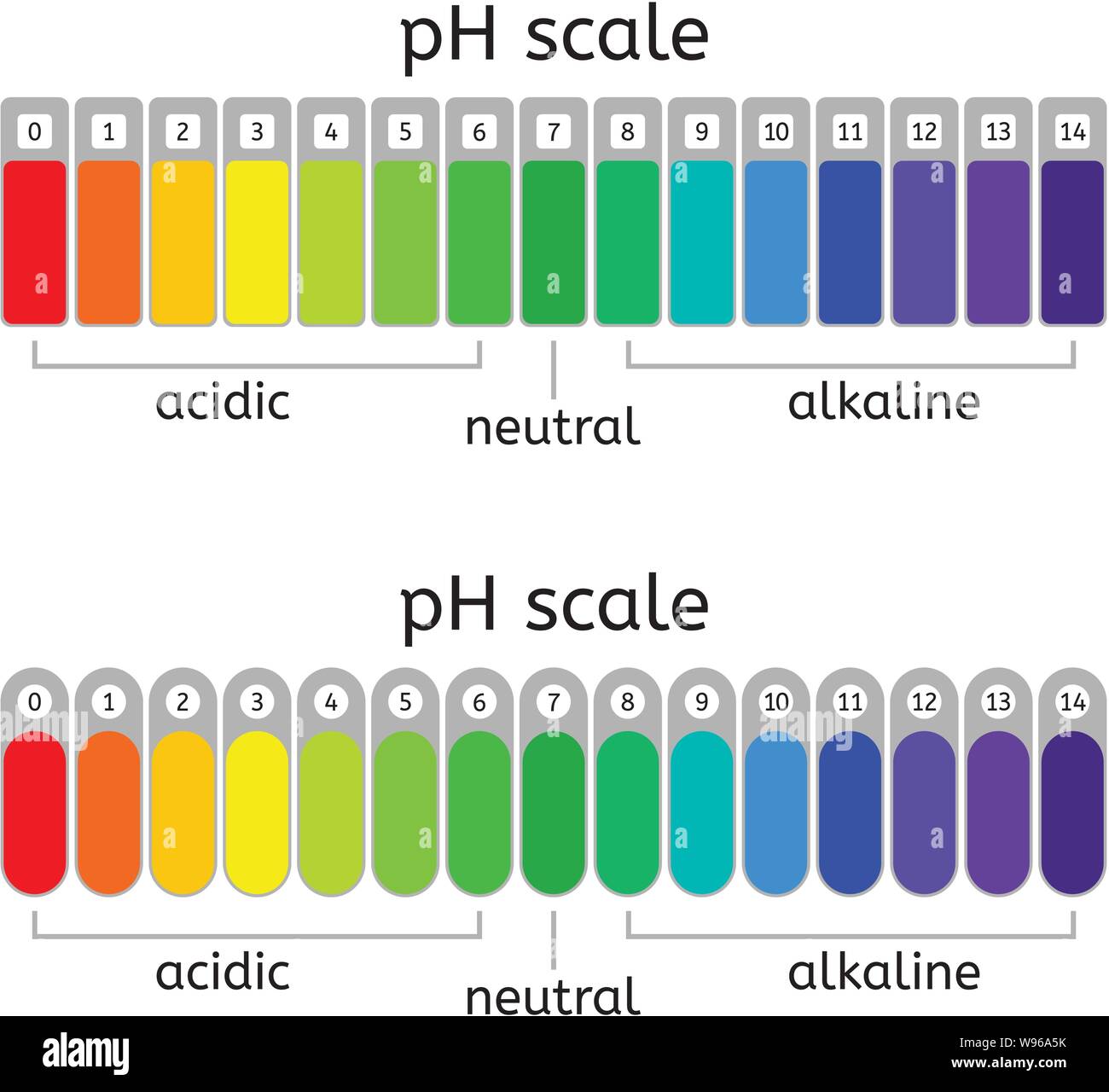
What Is A Buffer On The Ph Scale . The formula for ph is. what is a ph buffer? buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. It is able to neutralize. Ph is measured on a logarithmic scale. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
from www.vrogue.co
vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. what is a ph buffer? Ph is measured on a logarithmic scale. It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The formula for ph is. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a.
Ph Scale Value Infographic Acidbase Balance Scale For vrogue.co
What Is A Buffer On The Ph Scale Ph is measured on a logarithmic scale. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. what is a ph buffer? Ph is measured on a logarithmic scale. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. It is able to neutralize. The formula for ph is. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be.
From www.vrogue.co
Ph Scale Value Infographic Acidbase Balance Scale For vrogue.co What Is A Buffer On The Ph Scale what is a ph buffer? The formula for ph is. Ph is measured on a logarithmic scale. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. To be able to add a strong acid or base to a solution without causing a. What Is A Buffer On The Ph Scale.
From pressbooks.bccampus.ca
Unit 9 Fluids & Electrolytes Douglas College Human Anatomy What Is A Buffer On The Ph Scale buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture. What Is A Buffer On The Ph Scale.
From www.alamy.com
Diagram of the pH scale with examples of acidic, neutral and alkaline What Is A Buffer On The Ph Scale Ph is measured on a logarithmic scale. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. The formula for ph is. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. a buffer. What Is A Buffer On The Ph Scale.
From www.alamy.com
The pH scale Universal Indicator pH Color Chart diagram acidic Stock What Is A Buffer On The Ph Scale vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. The formula for ph is. what is a ph buffer? Ph is measured on a logarithmic scale. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice. What Is A Buffer On The Ph Scale.
From sciencesummative.wordpress.com
pH Scale sciencesummative What Is A Buffer On The Ph Scale The formula for ph is. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. Ph is measured. What Is A Buffer On The Ph Scale.
From mmerevise.co.uk
pH Curves Questions and Revision MME What Is A Buffer On The Ph Scale buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. It is able to neutralize. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. The formula for ph is. vertebrate organisms maintain the. What Is A Buffer On The Ph Scale.
From www.youtube.com
The pH Scale and Neutralisation for AQA 91 GCSE Chemistry and Trilogy What Is A Buffer On The Ph Scale It is able to neutralize. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Ph is measured on a logarithmic scale. what. What Is A Buffer On The Ph Scale.
From www.learner.org
The pH Scale (animation) Annenberg Learner What Is A Buffer On The Ph Scale Ph is measured on a logarithmic scale. It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is a ph buffer? To be able to add a strong acid or base to a solution without causing a large change in the ph, we. What Is A Buffer On The Ph Scale.
From preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories What Is A Buffer On The Ph Scale buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. It is able to neutralize. Ph is measured on a logarithmic scale. The formula for ph is. what is a ph buffer? vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid. What Is A Buffer On The Ph Scale.
From www.vrogue.co
Ph Scale Infographic Acidbase Balance Scale For Chemi vrogue.co What Is A Buffer On The Ph Scale Ph is measured on a logarithmic scale. The formula for ph is. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3). What Is A Buffer On The Ph Scale.
From www.youtube.com
How to Calculate PH of Buffer Solution YouTube What Is A Buffer On The Ph Scale a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The formula for ph is. It is able to neutralize. Ph is measured on a logarithmic scale. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph. What Is A Buffer On The Ph Scale.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub What Is A Buffer On The Ph Scale vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The formula for ph is. It is able to neutralize. buffers are solutions that moderate. What Is A Buffer On The Ph Scale.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is A Buffer On The Ph Scale buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets. What Is A Buffer On The Ph Scale.
From dxockvmtu.blob.core.windows.net
Ph Scale Definition Biology at Douglas Law blog What Is A Buffer On The Ph Scale Ph is measured on a logarithmic scale. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. what is a ph buffer? The formula for ph is. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of. What Is A Buffer On The Ph Scale.
From www.youtube.com
Calculating the pH of a buffer YouTube What Is A Buffer On The Ph Scale To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate. What Is A Buffer On The Ph Scale.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG What Is A Buffer On The Ph Scale vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. what is a ph buffer? buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. To be able to add a strong acid or base to. What Is A Buffer On The Ph Scale.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID297143 What Is A Buffer On The Ph Scale It is able to neutralize. what is a ph buffer? Ph is measured on a logarithmic scale. The formula for ph is. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffers are solutions that moderate ph changes when an acid or base is added to the. What Is A Buffer On The Ph Scale.
From kaiserscience.wordpress.com
pH scale « KaiserScience What Is A Buffer On The Ph Scale To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. Ph is measured on a logarithmic scale. The formula for ph is. It is able to neutralize. buffers are solutions that moderate ph changes when an acid or base is added to the. What Is A Buffer On The Ph Scale.
From courses.lumenlearning.com
pH, Buffers, Acids, and Bases Introduction to Chemistry What Is A Buffer On The Ph Scale It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is a ph buffer? vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. The formula for ph is.. What Is A Buffer On The Ph Scale.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Buffer On The Ph Scale vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base. What Is A Buffer On The Ph Scale.
From learningschooldsbbbb56.z4.web.core.windows.net
Explain The Ph Scale What Is A Buffer On The Ph Scale buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. The formula for ph is. what is a ph buffer? a buffer is. What Is A Buffer On The Ph Scale.
From www.etsy.com
Ph Scale Chart Print PDF Download Chemistry for Classroom Acid Alkaline What Is A Buffer On The Ph Scale A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. Ph is measured on a logarithmic scale. It is able to neutralize. The formula for ph is. what is a ph buffer? buffers are solutions that moderate ph changes when an acid. What Is A Buffer On The Ph Scale.
From www.differencebetween.com
Difference Between pH and Buffer Compare the Difference Between What Is A Buffer On The Ph Scale Ph is measured on a logarithmic scale. It is able to neutralize. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what. What Is A Buffer On The Ph Scale.
From mavink.com
Ph Scale Diagram What Is A Buffer On The Ph Scale what is a ph buffer? It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. A ph buffer. What Is A Buffer On The Ph Scale.
From saylordotorg.github.io
Buffers What Is A Buffer On The Ph Scale vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. Ph is measured on a logarithmic scale. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. To be able. What Is A Buffer On The Ph Scale.
From www.shutterstock.com
Bicarbonate Buffer Ph Scale Stock Vector (Royalty Free) 1839896080 What Is A Buffer On The Ph Scale It is able to neutralize. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Ph is measured on a logarithmic scale. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be. To be. What Is A Buffer On The Ph Scale.
From iastate.pressbooks.pub
Properties of Blood as a Buffer and Blood Glucose A Mixed Course What Is A Buffer On The Ph Scale what is a ph buffer? vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. The formula for ph. What Is A Buffer On The Ph Scale.
From content.byui.edu
Acids, Bases, pH and Buffers What Is A Buffer On The Ph Scale buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. It is able to neutralize. The formula for ph is. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Ph is measured on a logarithmic scale. vertebrate organisms maintain. What Is A Buffer On The Ph Scale.
From www.lecturio.com
Chemistry for Physicians Introduction Online Medical Library What Is A Buffer On The Ph Scale buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. what is a ph buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Ph is measured on a logarithmic scale. It is able to neutralize. To be able. What Is A Buffer On The Ph Scale.
From www.slideshare.net
Ph and buffer What Is A Buffer On The Ph Scale Ph is measured on a logarithmic scale. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize. A ph buffer is. What Is A Buffer On The Ph Scale.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog What Is A Buffer On The Ph Scale It is able to neutralize. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. The formula for ph is. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To be able to add a strong acid or base to. What Is A Buffer On The Ph Scale.
From www.slideserve.com
PPT Acids, Bases, & the pH Scale PowerPoint Presentation, free What Is A Buffer On The Ph Scale a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co. What Is A Buffer On The Ph Scale.
From www.youtube.com
Acids, Bases, pH, Buffers YouTube What Is A Buffer On The Ph Scale The formula for ph is. It is able to neutralize. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. A ph buffer is an aqueous solution consisting of a weak acid and its conjugate base or vice versa that lets the ph change to be.. What Is A Buffer On The Ph Scale.
From www.slideserve.com
PPT BIOCHEMISTRY PowerPoint Presentation, free download ID4746646 What Is A Buffer On The Ph Scale buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. To be able to add a strong acid or base to a solution without causing a large change in the ph, we need to create a. what is a ph buffer? The formula for ph is. a buffer is. What Is A Buffer On The Ph Scale.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube What Is A Buffer On The Ph Scale vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. what is a ph buffer? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To be able to add a strong acid or base. What Is A Buffer On The Ph Scale.