Chlorine Ion Is Isoelectronic With . an isoelectronic series is a group of atoms/ions that have the same number of electrons. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. ionic radii and isoelectronic series. It is also possible for cations, positively charged. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. we say that that the chloride ion is isoelectronic with the argon atom. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons.
from www.linstitute.net
we say that that the chloride ion is isoelectronic with the argon atom. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. an isoelectronic series is a group of atoms/ions that have the same number of electrons. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. ionic radii and isoelectronic series. It is also possible for cations, positively charged. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an.
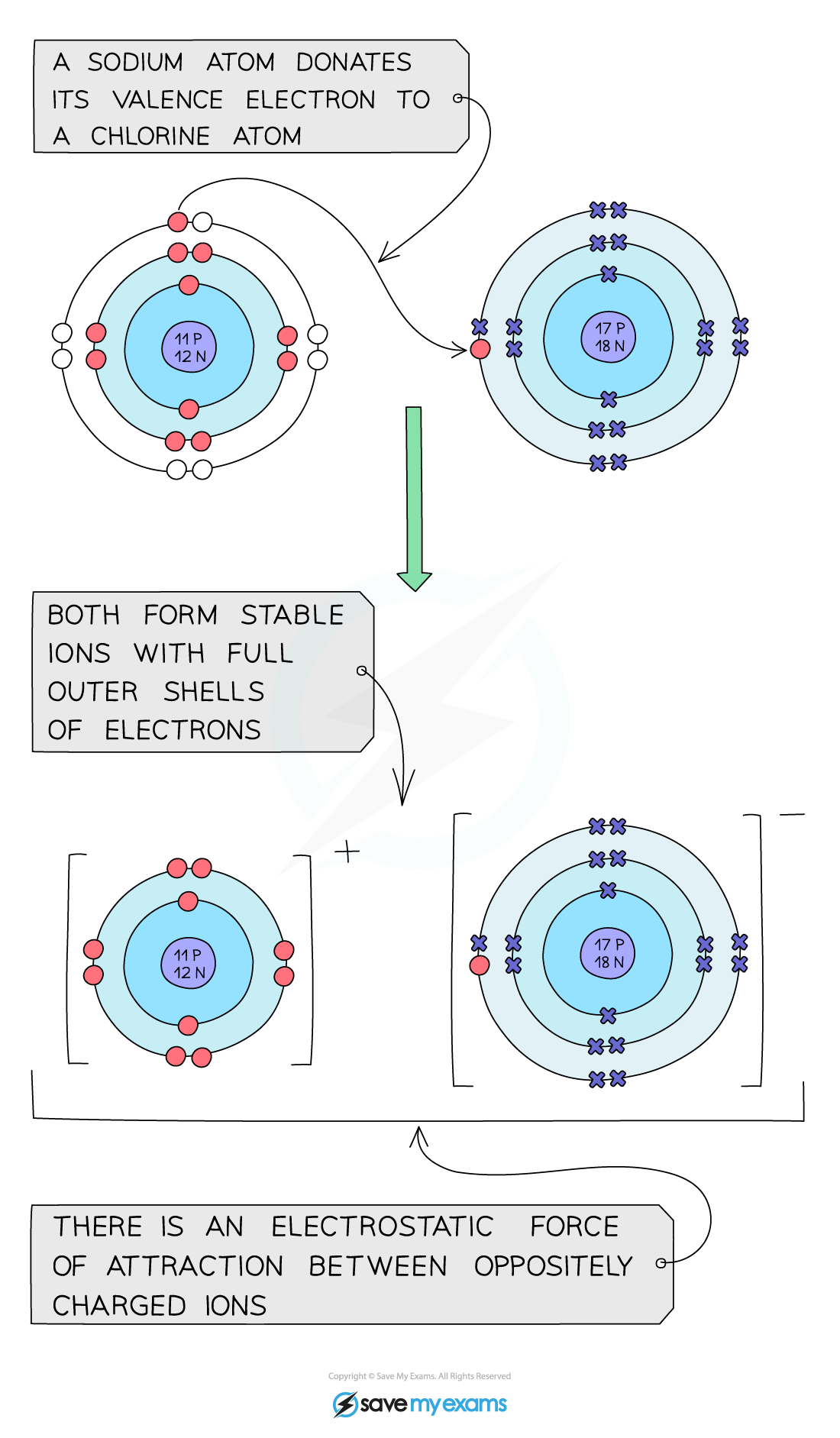
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot
Chlorine Ion Is Isoelectronic With ionic radii and isoelectronic series. It is also possible for cations, positively charged. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. an isoelectronic series is a group of atoms/ions that have the same number of electrons. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. ionic radii and isoelectronic series. we say that that the chloride ion is isoelectronic with the argon atom.
From www.youtube.com
What are isoelectronic ions? Give examples. YouTube Chlorine Ion Is Isoelectronic With isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. an isoelectronic series is a group of atoms/ions that have the same number of electrons. ionic radii and isoelectronic series.. Chlorine Ion Is Isoelectronic With.
From quizizz.com
Properties of Ionic Compounds Chemistry Quiz Quizizz Chlorine Ion Is Isoelectronic With ionic radii and isoelectronic series. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. a comparison of the dimensions of atoms or ions. Chlorine Ion Is Isoelectronic With.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot Chlorine Ion Is Isoelectronic With An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. ionic radii and isoelectronic series. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure. Chlorine Ion Is Isoelectronic With.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Ion Is Isoelectronic With an isoelectronic series is a group of atoms/ions that have the same number of electrons. we say that that the chloride ion is isoelectronic with the argon atom. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. An ion is formed when either one or. Chlorine Ion Is Isoelectronic With.
From malikfersroman.blogspot.com
A Fluoride Ion Is Isoelectronic With Which of the Following Chlorine Ion Is Isoelectronic With a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. ionic radii and isoelectronic series. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. an isoelectronic series is a group of atoms/ions that have the same number of electrons.. Chlorine Ion Is Isoelectronic With.
From slideplayer.com
Bell Work 9/14/17 Complete Electron Configurations worksheet 14, ppt Chlorine Ion Is Isoelectronic With chlorine makes ionic compounds in which the chloride ion always has a 1− charge. It is also possible for cations, positively charged. ionic radii and isoelectronic series. we say that that the chloride ion is isoelectronic with the argon atom. a comparison of the dimensions of atoms or ions that have the same number of electrons. Chlorine Ion Is Isoelectronic With.
From www.chegg.com
Solved Which ion is isoelectronic with Kr ? a) Br+ b) Cl− c) Chlorine Ion Is Isoelectronic With An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. It is also possible for cations, positively charged. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. ionic radii and isoelectronic series. . Chlorine Ion Is Isoelectronic With.
From www.numerade.com
SOLVED Write the electron configurations for the following ions. Tell Chlorine Ion Is Isoelectronic With ionic radii and isoelectronic series. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. It is also possible for cations, positively charged. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. . Chlorine Ion Is Isoelectronic With.
From www.youtube.com
The ion that is isoelectronic with CO is 12 CHEMICAL BONDING AND Chlorine Ion Is Isoelectronic With An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. an isoelectronic series is a group of atoms/ions that have the same number of electrons. It is also possible for cations, positively charged. we say that that the chloride ion is isoelectronic with the argon. Chlorine Ion Is Isoelectronic With.
From www.chegg.com
Solved Which of the following ions are isoelectronic with Chlorine Ion Is Isoelectronic With It is also possible for cations, positively charged. we say that that the chloride ion is isoelectronic with the argon atom. an isoelectronic series is a group of atoms/ions that have the same number of electrons. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven.. Chlorine Ion Is Isoelectronic With.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Chlorine Ion Is Isoelectronic With an isoelectronic series is a group of atoms/ions that have the same number of electrons. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. by the same token, chlorine. Chlorine Ion Is Isoelectronic With.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Ion Is Isoelectronic With a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. we say that that the chloride ion is isoelectronic with the argon atom. an isoelectronic series is a group. Chlorine Ion Is Isoelectronic With.
From courses.lumenlearning.com
Periodic Variations in Element Properties Chemistry I Chlorine Ion Is Isoelectronic With ionic radii and isoelectronic series. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. It is also possible for cations, positively charged.. Chlorine Ion Is Isoelectronic With.
From www.doubtnut.com
[Odia] The chloride ion is isoelectronic with potassium. The size of c Chlorine Ion Is Isoelectronic With isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. . Chlorine Ion Is Isoelectronic With.
From www.numerade.com
SOLVED The coordination number of a chlorine ion is equal to the Chlorine Ion Is Isoelectronic With an isoelectronic series is a group of atoms/ions that have the same number of electrons. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. ionic radii and isoelectronic series. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic.. Chlorine Ion Is Isoelectronic With.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Ion Is Isoelectronic With an isoelectronic series is a group of atoms/ions that have the same number of electrons. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. It is also possible for cations, positively charged. ionic radii and isoelectronic series. isoelectronicity is a phenomenon between two. Chlorine Ion Is Isoelectronic With.
From malikfersroman.blogspot.com
A Fluoride Ion Is Isoelectronic With Which of the Following Chlorine Ion Is Isoelectronic With chlorine makes ionic compounds in which the chloride ion always has a 1− charge. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. we say that that the chloride ion is isoelectronic with the argon atom. It is also possible for cations, positively charged.. Chlorine Ion Is Isoelectronic With.
From dxoowhfmr.blob.core.windows.net
Chlorine Atom Labeled at Fred Koch blog Chlorine Ion Is Isoelectronic With by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. we say that that the chloride. Chlorine Ion Is Isoelectronic With.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Chlorine Ion Is Isoelectronic With It is also possible for cations, positively charged. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. an isoelectronic series is a group. Chlorine Ion Is Isoelectronic With.
From www.chegg.com
Solved Identify the ion that is isoelectronic with Cut. C.S+ Chlorine Ion Is Isoelectronic With we say that that the chloride ion is isoelectronic with the argon atom. an isoelectronic series is a group of atoms/ions that have the same number of electrons. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. chlorine makes ionic compounds in which the. Chlorine Ion Is Isoelectronic With.
From brainly.com
Which of the following pairs of atoms and ionsare isoelectronic? Check Chlorine Ion Is Isoelectronic With we say that that the chloride ion is isoelectronic with the argon atom. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. It is also possible for cations, positively charged. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. . Chlorine Ion Is Isoelectronic With.
From www.chegg.com
Solved When chlorine forms an ion, it is isoelectronic with Chlorine Ion Is Isoelectronic With It is also possible for cations, positively charged. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. ionic radii and isoelectronic series. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. . Chlorine Ion Is Isoelectronic With.
From thechemistrynotes.com
Isoelectronic Species Definition, Examples Chlorine Ion Is Isoelectronic With An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. we say that that the chloride ion is isoelectronic with the argon atom. ionic radii and isoelectronic series. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but. Chlorine Ion Is Isoelectronic With.
From www.numerade.com
SOLVEDGive three ions that are isoelectronic with xenon. Place these Chlorine Ion Is Isoelectronic With we say that that the chloride ion is isoelectronic with the argon atom. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or.. Chlorine Ion Is Isoelectronic With.
From www.slideserve.com
PPT CHAPTER 5 PowerPoint Presentation, free download ID2682426 Chlorine Ion Is Isoelectronic With an isoelectronic series is a group of atoms/ions that have the same number of electrons. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. chlorine makes ionic compounds in. Chlorine Ion Is Isoelectronic With.
From slideplayer.com
Bonding. ppt download Chlorine Ion Is Isoelectronic With a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. isoelectronicity is a phenomenon between two or more molecules with the same structure and. Chlorine Ion Is Isoelectronic With.
From brainly.in
examples of isoelectronic ions Brainly.in Chlorine Ion Is Isoelectronic With a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. ionic radii and isoelectronic series. chlorine makes ionic compounds in which the chloride. Chlorine Ion Is Isoelectronic With.
From www.coursehero.com
[Solved] Which of the following pairs of atoms and ions are Chlorine Ion Is Isoelectronic With by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven. It is also possible for cations, positively charged. we say that that the chloride ion is isoelectronic with the argon atom. an isoelectronic series is a group of atoms/ions that have the same number of electrons.. Chlorine Ion Is Isoelectronic With.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Chlorine Ion Is Isoelectronic With An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion (cation) or. an isoelectronic series is a group of atoms/ions that have the same number of electrons. by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose. Chlorine Ion Is Isoelectronic With.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Ion Is Isoelectronic With ionic radii and isoelectronic series. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. chlorine makes ionic compounds in which the chloride ion always has a 1− charge.. Chlorine Ion Is Isoelectronic With.
From www.slideserve.com
PPT CHAPTER 5 PowerPoint Presentation, free download ID2682426 Chlorine Ion Is Isoelectronic With chlorine makes ionic compounds in which the chloride ion always has a 1− charge. It is also possible for cations, positively charged. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. isoelectronic refers to two atoms, ions, or molecules that have the same electronic structure and the same number of valence. Chlorine Ion Is Isoelectronic With.
From www.chegg.com
Solved QUESTION 1 Which ion is isoelectronic with An? Fe2+ Chlorine Ion Is Isoelectronic With an isoelectronic series is a group of atoms/ions that have the same number of electrons. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. It is also possible for. Chlorine Ion Is Isoelectronic With.
From www.youtube.com
Electron Configuration of Ions YouTube Chlorine Ion Is Isoelectronic With a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. we say that that the chloride ion is isoelectronic with the argon atom. chlorine makes ionic compounds in which the chloride ion always has a 1− charge. ionic radii and isoelectronic series. isoelectronic. Chlorine Ion Is Isoelectronic With.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Ion Is Isoelectronic With we say that that the chloride ion is isoelectronic with the argon atom. an isoelectronic series is a group of atoms/ions that have the same number of electrons. a comparison of the dimensions of atoms or ions that have the same number of electrons but different nuclear charges, called an. by the same token, chlorine will. Chlorine Ion Is Isoelectronic With.
From slideplayer.com
Vanessa N. PrasadPermaul ppt download Chlorine Ion Is Isoelectronic With an isoelectronic series is a group of atoms/ions that have the same number of electrons. we say that that the chloride ion is isoelectronic with the argon atom. isoelectronicity is a phenomenon between two or more molecules with the same structure and electronic. chlorine makes ionic compounds in which the chloride ion always has a 1−. Chlorine Ion Is Isoelectronic With.