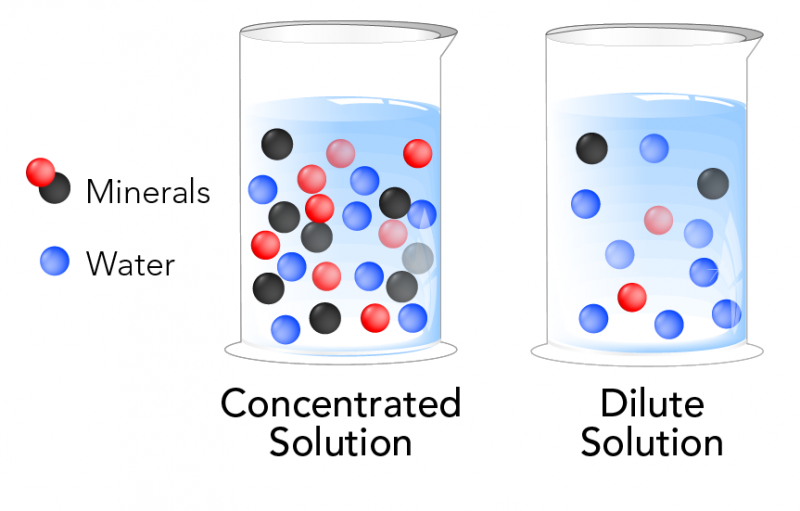
Dilute Example Science . Understand how stock solutions are used in the laboratory. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. diluting a sample or solution is common practice in science. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also.
from www.aiophotoz.com
diluting a sample or solution is common practice in science. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Understand how stock solutions are used in the laboratory. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. explain how concentrations can be changed in the lab.
What Is The Difference Between Dilute And Concentrated Solution
Dilute Example Science a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. diluting a sample or solution is common practice in science. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. learn how to dilute and concentrate solutions.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Example Science diluting a sample or solution is common practice in science. learn how to dilute and concentrate solutions. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Often, a. Dilute Example Science.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilute Example Science Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Understand how stock solutions are used in the laboratory. the dilution equation is a simple relation between concentrations and volumes of a solution. Dilute Example Science.
From www.youtube.com
pH of Dilute Solution (Example) YouTube Dilute Example Science a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. explain how concentrations can be changed in the lab. dilution is the process. Dilute Example Science.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Example Science dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. a dilution can be. Dilute Example Science.
From solanolabs.com
Saturated solution definition chemistry Difference Between Saturated Dilute Example Science a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. diluting a sample or solution is common practice in science. . Dilute Example Science.
From sciencenotes.org
Dilution Science Notes and Projects Dilute Example Science diluting a sample or solution is common practice in science. Understand how stock solutions are used in the laboratory. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. . Dilute Example Science.
From www.pinterest.com.mx
Example of a Serial Dilution Medical laboratory science, Medical Dilute Example Science learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. explain how concentrations can be changed in. Dilute Example Science.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilute Example Science learn how to dilute and concentrate solutions. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. a dilution is a process where the concentration of a solution is lowered by. Dilute Example Science.
From labpedia.net
Solutions Part 3 Solutions with various examples Dilute Example Science learn how to dilute and concentrate solutions. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Understand how stock solutions are used in the laboratory. diluting a sample or solution is common practice in science. Often, a worker will need to change the concentration of a solution by changing. Dilute Example Science.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilute Example Science dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. the dilution equation is a simple relation between concentrations and volumes of a solution before. Dilute Example Science.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog Dilute Example Science learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but. Dilute Example Science.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilute Example Science diluting a sample or solution is common practice in science. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. explain how concentrations can be. Dilute Example Science.
From www.showme.com
Concentrated vs. Diluted Solution Science ShowMe Dilute Example Science explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. diluting a sample or solution is common practice in science. Often, a worker will need to change the concentration of a solution by. Dilute Example Science.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog Dilute Example Science a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution. Dilute Example Science.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Example Science a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. the dilution equation is a simple relation between concentrations and volumes of a solution before. Dilute Example Science.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Example Science a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. diluting a sample or solution is common practice in science. the dilution equation is. Dilute Example Science.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilute Example Science diluting a sample or solution is common practice in science. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. explain how concentrations can be changed in the. Dilute Example Science.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Example Science a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. diluting. Dilute Example Science.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Example Science a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. a dilution can be used to not only lower the concentration of the analyte being tested so that it is. Dilute Example Science.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Example Science a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. dilution is the process of making a solution or scientific sample less. Dilute Example Science.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Example Science Often, a worker will need to change the concentration of a solution by changing the amount of solvent. explain how concentrations can be changed in the lab. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. diluting a sample or solution is common practice in science. the dilution. Dilute Example Science.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Example Science learn how to dilute and concentrate solutions. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Understand how stock solutions are used in the laboratory. a dilution is a process. Dilute Example Science.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilute Example Science explain how concentrations can be changed in the lab. diluting a sample or solution is common practice in science. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are used in the laboratory. learn how to dilute and concentrate solutions. a dilution. Dilute Example Science.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Example Science Understand how stock solutions are used in the laboratory. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. explain how concentrations can be changed in the lab. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Often, a. Dilute Example Science.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilute Example Science explain how concentrations can be changed in the lab. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. the dilution. Dilute Example Science.
From ar.inspiredpencil.com
Dilute Science Dilute Example Science learn how to dilute and concentrate solutions. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. Understand how stock solutions are used in the laboratory. a dilution is a process where the concentration of a solution is lowered by adding solvent. Dilute Example Science.
From www.aiophotoz.com
What Is The Difference Between Dilute And Concentrated Solution Dilute Example Science diluting a sample or solution is common practice in science. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Often, a worker will need. Dilute Example Science.
From www.thoughtco.com
What Is the Definition of Dilute? Dilute Example Science diluting a sample or solution is common practice in science. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. the dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Understand how stock solutions are used in the laboratory. dilution is. Dilute Example Science.
From brainly.in
how can a solution be dilute or concentrated Brainly.in Dilute Example Science learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. diluting a sample or solution is common practice in science. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. the dilution equation is a simple relation between concentrations and volumes of a. Dilute Example Science.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Dilute Example Science Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. the. Dilute Example Science.
From www.slideshare.net
Serial dilution Dilute Example Science learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. explain how concentrations can be changed in the lab. the dilution equation is a simple. Dilute Example Science.
From fphoto.photoshelter.com
science chemistry molarity solution dilution Fundamental Photographs Dilute Example Science explain how concentrations can be changed in the lab. diluting a sample or solution is common practice in science. Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. dilution is the process of making a solution or scientific sample. Dilute Example Science.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilute Example Science Understand how stock solutions are used in the laboratory. dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. diluting a sample or solution is common practice in science. Often,. Dilute Example Science.
From sciencestruck.com
Here's How to Calculate Dilution Factor from Given Concentration Dilute Example Science learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. diluting a sample or solution is common practice in science. the dilution equation is a. Dilute Example Science.
From www.shutterstock.com
228 Dilute Solution Concentrated Solution Images, Stock Photos Dilute Example Science explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. a dilution can be used to not only lower the concentration of the analyte being tested so that it is below acceptable limits but also. diluting a sample or solution is common practice in science. Understand how stock solutions are. Dilute Example Science.