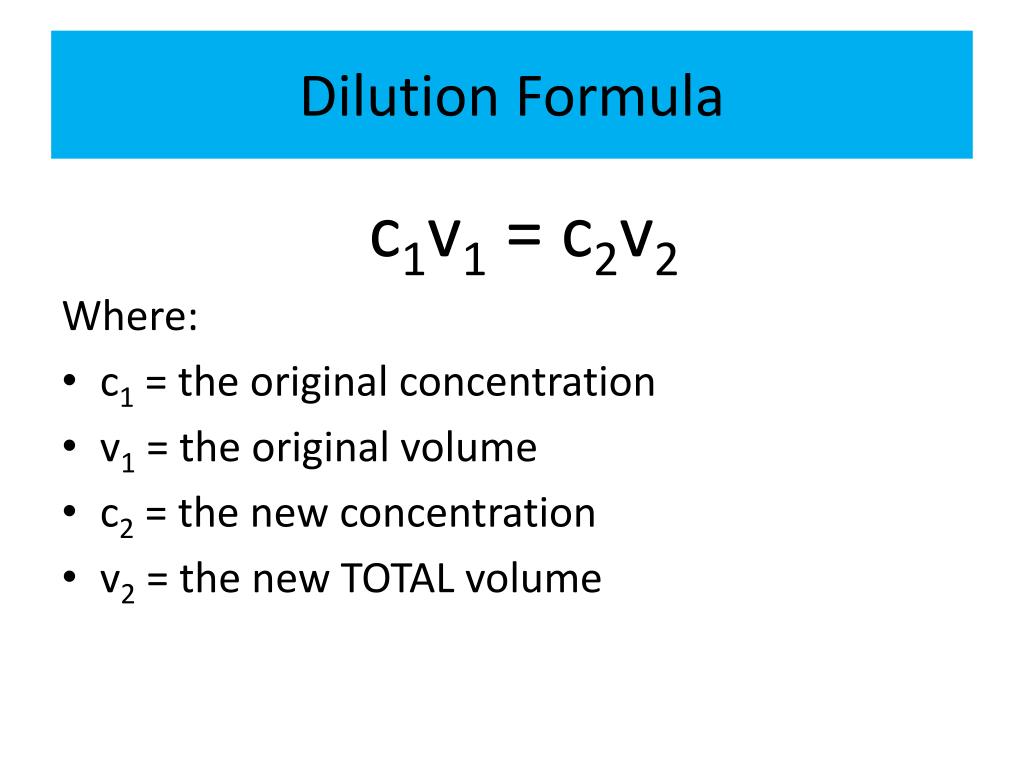
Dilution Of Solution . Dilution is a process used to lower the concentration of the original solution by adding more solvent. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. By the end of this section, you will be able to: Describe the fundamental properties of solutions. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). learn how to accurately dilute solutions with this simple guide.
from dxocmttnm.blob.core.windows.net
Describe the fundamental properties of solutions. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. learn how to accurately dilute solutions with this simple guide. By the end of this section, you will be able to: a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent.
Dilution Equation Formulas at Kathleen Milford blog
Dilution Of Solution By the end of this section, you will be able to: the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent. learn how to accurately dilute solutions with this simple guide. Describe the fundamental properties of solutions. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). By the end of this section, you will be able to:
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Of Solution By the end of this section, you will be able to: learn how to accurately dilute solutions with this simple guide. Dilution is a process used to lower the concentration of the original solution by adding more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or. Dilution Of Solution.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Of Solution By the end of this section, you will be able to: a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to accurately dilute solutions with this simple guide. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent.. Dilution Of Solution.
From spmchemistry.blog.onlinetuition.com.my
Dilution SPM Chemistry Dilution Of Solution learn how to accurately dilute solutions with this simple guide. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Dilution is a process used to lower the concentration of the. Dilution Of Solution.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of Dilution Of Solution a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is a process used to lower the concentration of the original solution by adding more solvent.. Dilution Of Solution.
From exoxgkatn.blob.core.windows.net
Dilute Solution Acid at Michael Keller blog Dilution Of Solution By the end of this section, you will be able to: the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Describe the fundamental properties of solutions. Dilution is a process used. Dilution Of Solution.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Of Solution Describe the fundamental properties of solutions. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to accurately dilute solutions with this simple guide. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. By the end of this. Dilution Of Solution.
From www.myxxgirl.com
Serial Dilution Of An Initial Sample Or Culture To Obtain Solutions Dilution Of Solution a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. learn how to accurately dilute solutions with this simple guide. Describe the fundamental properties of solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. a solution containing a precise. Dilution Of Solution.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of solutions. By the end of this section, you will be able to: Dilution is a process used to lower the concentration of the original solution by adding more solvent. learn how to accurately dilute solutions with. Dilution Of Solution.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Of Solution learn how to accurately dilute solutions with this simple guide. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). By the end of this section, you will be able to: the solution on the right is more dilute because the copper nitrate is dissolved. Dilution Of Solution.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of solutions. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilute solution is one in which there is a relatively small. Dilution Of Solution.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Of Solution Dilution is a process used to lower the concentration of the original solution by adding more solvent. Describe the fundamental properties of solutions. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. the solution on the right is more dilute because the copper nitrate is dissolved in more. Dilution Of Solution.
From studylib.net
Key DilutionsWorksheet Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the. Dilution Of Solution.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Of Solution Dilution is a process used to lower the concentration of the original solution by adding more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of. Dilution Of Solution.
From www.chegg.com
Solved In The Image Above, The Final Dilution Is 1 You M... Dilution Of Solution a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of solutions. a solution containing a precise mass of solute in a precise volume of solution is. Dilution Of Solution.
From www.chegg.com
Solved Serial dilution is a common technique used in Dilution Of Solution By the end of this section, you will be able to: the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Dilution is a process used to lower the. Dilution Of Solution.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). learn how to accurately dilute solutions with this simple guide. a dilute solution is one in which. Dilution Of Solution.
From www.pdfprof.com
PDF parts dilution calculator PDF Télécharger Download Dilution Of Solution Describe the fundamental properties of solutions. By the end of this section, you will be able to: Dilution is a process used to lower the concentration of the original solution by adding more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. a solution containing a precise. Dilution Of Solution.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Describe the fundamental properties of solutions. a solution containing a precise mass of solute in a precise volume of solution is. Dilution Of Solution.
From mfawriting332.web.fc2.com
How do you calculate dilution? Dilution Of Solution a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. learn how to accurately dilute solutions with this simple guide. Describe the fundamental properties of solutions. a. Dilution Of Solution.
From studylib.net
Solutions and Dilutions Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of solutions. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilute solution is one in which there is a relatively small. Dilution Of Solution.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Of Solution learn how to accurately dilute solutions with this simple guide. By the end of this section, you will be able to: the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Dilution is a process used to lower the concentration of the original solution by adding more solvent. a solution. Dilution Of Solution.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Of Solution a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Dilution is a process used to lower the concentration of the original solution by adding more solvent. Describe the fundamental properties of solutions. By the end of this section, you will be able to: a dilute. Dilution Of Solution.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution Of Solution learn how to accurately dilute solutions with this simple guide. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). By the end of this section,. Dilution Of Solution.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. learn how to accurately dilute solutions with this simple guide. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a dilute solution is one in which. Dilution Of Solution.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Of Solution learn how to accurately dilute solutions with this simple guide. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Dilution is a process used to lower the. Dilution Of Solution.
From sciencesavers.info
System, Calculator, Methodology, Makes use of, Examples sciencesavers Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. By the end of this section, you will be able to: a dilute solution is one in which. Dilution Of Solution.
From sciencestruck.com
Here's How to Calculate Dilution Factor from Given Concentration Dilution Of Solution By the end of this section, you will be able to: Dilution is a process used to lower the concentration of the original solution by adding more solvent. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of solutions. learn how to accurately dilute solutions with. Dilution Of Solution.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog Dilution Of Solution learn how to accurately dilute solutions with this simple guide. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Dilution is a process used to lower the. Dilution Of Solution.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Of Solution Describe the fundamental properties of solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). learn how to accurately dilute solutions with this simple guide. By the end. Dilution Of Solution.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. learn how to accurately dilute solutions with this simple guide. By the end of this section, you will be able to: a solution containing a precise mass of solute in a precise volume of solution is called a stock solution. Dilution Of Solution.
From socratic.org
How many milliliters of 12.0 M HCl(aq) must be diluted with water to Dilution Of Solution learn how to accurately dilute solutions with this simple guide. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. Describe the fundamental properties of solutions. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. a solution containing a. Dilution Of Solution.
From americanlaboratory.com
Direct Improvement With Direct Dilution American Laboratory Dilution Of Solution Dilution is a process used to lower the concentration of the original solution by adding more solvent. Describe the fundamental properties of solutions. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. a solution containing a precise mass of solute in a precise volume of solution is called. Dilution Of Solution.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Of Solution learn how to accurately dilute solutions with this simple guide. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Dilution is a process used to lower the. Dilution Of Solution.
From www.youtube.com
Stock Solution Dilutions Dilution Calculation [Learn how to make any Dilution Of Solution the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). Describe the fundamental properties of solutions. a dilute solution is one in which there is a relatively small. Dilution Of Solution.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution Of Solution Describe the fundamental properties of solutions. learn how to accurately dilute solutions with this simple guide. the solution on the right is more dilute because the copper nitrate is dissolved in more solvent. a solution containing a precise mass of solute in a precise volume of solution is called a stock solution (or standard solution). a. Dilution Of Solution.