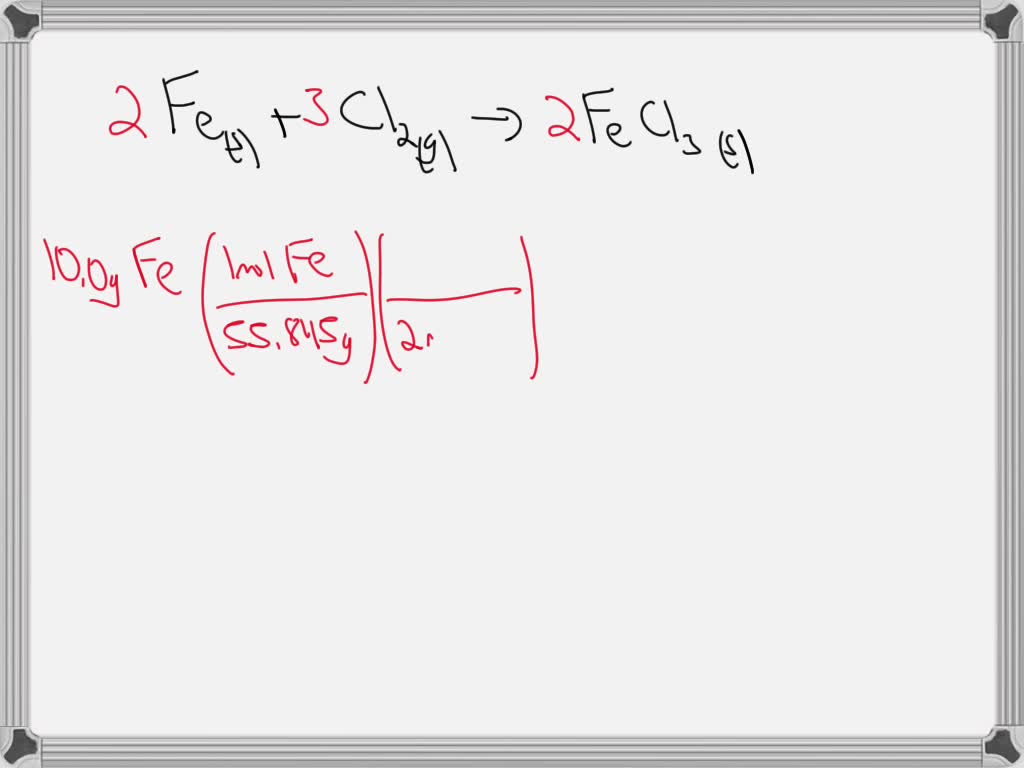Iron Iii Chloride Water Reaction . Preparation of ferric chloride solution. The chemical equation for this reaction is provided below. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). Its high solubility and ability to form complexes make it effective in removing impurities and. The chemistry of the (etching) reaction. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. In the realm of water treatment, iron(iii) chloride plays a pivotal role. 2fe + 3cl 2 → 2fecl 3. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride.
from www.numerade.com
In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. 2fe + 3cl 2 → 2fecl 3. In the realm of water treatment, iron(iii) chloride plays a pivotal role. Preparation of ferric chloride solution. Its high solubility and ability to form complexes make it effective in removing impurities and. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. The chemistry of the (etching) reaction. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii).
SOLVED In the reaction to form iron(III) chloride, 10.0 grams of iron
Iron Iii Chloride Water Reaction Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. 2fe + 3cl 2 → 2fecl 3. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. The chemistry of the (etching) reaction. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. The chemical equation for this reaction is provided below. Its high solubility and ability to form complexes make it effective in removing impurities and. In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water. In the realm of water treatment, iron(iii) chloride plays a pivotal role. Preparation of ferric chloride solution.
From www.youtube.com
Chemistry 110, Experiment 15 Part Two Oxidation of Alcohols, Iron Iron Iii Chloride Water Reaction Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. 2fe + 3cl 2 → 2fecl 3. Its high solubility and ability to form complexes make it effective in removing impurities and. In the realm of water treatment, iron(iii) chloride plays a pivotal role. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron. Iron Iii Chloride Water Reaction.
From www.sciencephoto.com
Sodium hydroxide and iron III chloride Stock Image C028/4185 Iron Iii Chloride Water Reaction The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water. Preparation of ferric chloride solution. Its high solubility and ability to form complexes make it effective in removing impurities and. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. Cationic hydrolysis is possible because the salt, iron (iii). Iron Iii Chloride Water Reaction.
From www.researchgate.net
Raman spectra of iron(III) chloride in the solid, liquid and gaseous Iron Iii Chloride Water Reaction Its high solubility and ability to form complexes make it effective in removing impurities and. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does. Iron Iii Chloride Water Reaction.
From www.researchgate.net
What is the chemical difference between these iron III chloride Iron Iii Chloride Water Reaction When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. The chemical equation for this reaction is provided below. Preparation of ferric chloride solution. The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water. Its high solubility and ability to form complexes make it effective in removing. Iron Iii Chloride Water Reaction.
From www.alamy.com
Iron (III) hydroxide precipitation. Test tube containing sodium Iron Iii Chloride Water Reaction Its high solubility and ability to form complexes make it effective in removing impurities and. Preparation of ferric chloride solution. 2fe + 3cl 2 → 2fecl 3. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2. Iron Iii Chloride Water Reaction.
From www.sciencephoto.com
Iron (III) oxide in water and in hydrochloric acid Stock Image C036 Iron Iii Chloride Water Reaction When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. The chemical equation for this reaction is provided below. The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water. In the realm of water treatment, iron(iii) chloride plays a pivotal role. By dissolving iron ore in hcl (hydrochloric acid). Iron Iii Chloride Water Reaction.
From www.numerade.com
SOLVEDThe reaction of iron metal and chlorine gas to give iron(III Iron Iii Chloride Water Reaction By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. Preparation of. Iron Iii Chloride Water Reaction.
From www.numerade.com
SOLVEDIron(III) chloride is produced by the reaction of iron and Iron Iii Chloride Water Reaction When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. The chemical equation for this reaction is provided below. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. 2fe + 3cl 2. Iron Iii Chloride Water Reaction.
From stock.adobe.com
Chemical reactionPipette 0.25 M solution of iron(III) chloride (FeCl3 Iron Iii Chloride Water Reaction By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. The chemical equation for this reaction is provided below. 2fe + 3cl 2 → 2fecl 3. Preparation of ferric chloride solution. In the realm of water treatment, iron(iii) chloride plays a pivotal role. The chemistry of. Iron Iii Chloride Water Reaction.
From www.youtube.com
How to Write the Formula for Iron (III) chloride YouTube Iron Iii Chloride Water Reaction In the realm of water treatment, iron(iii) chloride plays a pivotal role. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. The chemical equation for this reaction is provided below. Cationic hydrolysis is possible because the salt, iron. Iron Iii Chloride Water Reaction.
From www.coursehero.com
[Solved] When aqueous an solutions of iron(III) sulfate and sodium Iron Iii Chloride Water Reaction Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. The chemistry of the (etching) reaction. Preparation of ferric chloride solution. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. Cationic hydrolysis is. Iron Iii Chloride Water Reaction.
From www.chegg.com
Solved (4pts) For the reaction of iron(III) chloride and Iron Iii Chloride Water Reaction Its high solubility and ability to form complexes make it effective in removing impurities and. Preparation of ferric chloride solution. 2fe + 3cl 2 → 2fecl 3. In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. The chemical. Iron Iii Chloride Water Reaction.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Iron Iii Chloride Water Reaction When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring. Iron Iii Chloride Water Reaction.
From www.chegg.com
Solved Reaction 10 Aqueous iron(III) chloride + aqueous Iron Iii Chloride Water Reaction When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. The chemistry of the (etching) reaction. In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. The chemical equation for this reaction is. Iron Iii Chloride Water Reaction.
From www.youtube.com
Equation for FeCl3 + H2O Iron (III) chloride + Water YouTube Iron Iii Chloride Water Reaction Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. The chemistry of the (etching) reaction. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring. Iron Iii Chloride Water Reaction.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Chloride Water Reaction By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. The chemical equation for this reaction is provided below. Its high solubility and ability to form complexes make it effective in removing impurities and. The chemistry of the (etching) reaction. Iron(iii) chloride indeed hydrolyses in water,. Iron Iii Chloride Water Reaction.
From www.numerade.com
SOLVED 90.0 g of iron (III) chloride reacts with 52.0 g of hydrogen Iron Iii Chloride Water Reaction The chemical equation for this reaction is provided below. 2fe + 3cl 2 → 2fecl 3. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. By dissolving iron ore in hcl (hydrochloric acid) fe 3. Iron Iii Chloride Water Reaction.
From www.researchgate.net
What is the chemical difference between these iron III chloride Iron Iii Chloride Water Reaction In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. The chemistry of the (etching) reaction. In the realm of water treatment, iron(iii) chloride plays a pivotal role.. Iron Iii Chloride Water Reaction.
From www.numerade.com
SOLVEDIn water, iron(III) chloride reacts with sodium hydroxide Iron Iii Chloride Water Reaction In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. The chemistry of the (etching) reaction. Preparation of ferric chloride solution. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. 2fe + 3cl 2 → 2fecl 3. The chemical equation for this reaction. Iron Iii Chloride Water Reaction.
From www.numerade.com
SOLVED In the reaction to form iron(III) chloride, 10.0 grams of iron Iron Iii Chloride Water Reaction The chemistry of the (etching) reaction. In the realm of water treatment, iron(iii) chloride plays a pivotal role. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. Its high solubility and ability to form complexes make it effective in removing impurities and. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic. Iron Iii Chloride Water Reaction.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron Iii Chloride Water Reaction Its high solubility and ability to form complexes make it effective in removing impurities and. The chemistry of the (etching) reaction. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water.. Iron Iii Chloride Water Reaction.
From www.youtube.com
How to write the formula for iron (III) chloride YouTube Iron Iii Chloride Water Reaction Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. The chemical equation for this reaction is provided below. In this video we will describe the equation fecl3 + h2o and. Iron Iii Chloride Water Reaction.
From www.youtube.com
Reaction of Iron (III) chloride (FeCl3) with Ammonium hydroxide (NH4OH Iron Iii Chloride Water Reaction In the realm of water treatment, iron(iii) chloride plays a pivotal role. The chemical equation for this reaction is provided below. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. By dissolving iron ore in. Iron Iii Chloride Water Reaction.
From www.youtube.com
Iron (III) Chloride Dissolution in Water Animated Reaction YouTube Iron Iii Chloride Water Reaction 2fe + 3cl 2 → 2fecl 3. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). In the realm of water treatment, iron(iii) chloride plays a pivotal role.. Iron Iii Chloride Water Reaction.
From www.youtube.com
Does Iron (III) Chloride/FeCl3 react with aspirin or salicylic acid Iron Iii Chloride Water Reaction In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). The chemistry of the (etching) reaction. 2fe + 3cl 2 → 2fecl 3. Preparation of ferric chloride solution. Anhydrous iron (iii) chloride can be prepared. Iron Iii Chloride Water Reaction.
From icsechemistry16.blogspot.com
Preparation of Iron (III) chloride (Ferric chloride) Iron Iii Chloride Water Reaction The chemical equation for this reaction is provided below. Its high solubility and ability to form complexes make it effective in removing impurities and. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. By dissolving iron ore in. Iron Iii Chloride Water Reaction.
From www.chegg.com
Solved When iron (III) chloride and hydrogen sulfide react Iron Iii Chloride Water Reaction When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. Its high solubility and ability to form complexes make it effective in removing impurities and. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). The chemical equation for this reaction is provided below. The chemistry of. Iron Iii Chloride Water Reaction.
From www.youtube.com
Iron III Chloride Reaction With Potassium Thiocyanate (FeCl3 + KSCN Iron Iii Chloride Water Reaction The chemistry of the (etching) reaction. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. The chemical equation for this reaction is provided below. Preparation of ferric chloride solution. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. Its high solubility. Iron Iii Chloride Water Reaction.
From www.youtube.com
The reaction of iron(III) solution with sodium hydroxide and ammonia Iron Iii Chloride Water Reaction The chemistry of the (etching) reaction. In the realm of water treatment, iron(iii) chloride plays a pivotal role. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. In this. Iron Iii Chloride Water Reaction.
From www.numerade.com
SOLVED In water, iron(III) chloride reacts with sodium hydroxide Iron Iii Chloride Water Reaction 2fe + 3cl 2 → 2fecl 3. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. The chemistry of the (etching). Iron Iii Chloride Water Reaction.
From www.youtube.com
Iron(iii) Chloride Preparation YouTube Iron Iii Chloride Water Reaction In the realm of water treatment, iron(iii) chloride plays a pivotal role. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. The hydrolysis of iron (iii) chloride is the cationic reaction of the salt with water. Preparation of ferric chloride solution. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a. Iron Iii Chloride Water Reaction.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron Iii Chloride Water Reaction Preparation of ferric chloride solution. When dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). When ferric chloride is dissolved in water the solution becomes strongly acidic as a result of. The chemistry of the. Iron Iii Chloride Water Reaction.
From www.slideserve.com
PPT Double Replacement Reactions PowerPoint Presentation, free Iron Iii Chloride Water Reaction Preparation of ferric chloride solution. Its high solubility and ability to form complexes make it effective in removing impurities and. The chemistry of the (etching) reaction. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. When dissolved in water, it undergoes hydrolysis, resulting in the. Iron Iii Chloride Water Reaction.
From www.chegg.com
Solved 2. Iron (III) chloride dissolves in water forming Iron Iii Chloride Water Reaction Preparation of ferric chloride solution. Cationic hydrolysis is possible because the salt, iron (iii) chloride, formed by a weak base (iron (iii). In this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron. Iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. Its high solubility and ability. Iron Iii Chloride Water Reaction.
From www.youtube.com
Iron(III) Thiocyanate Equilibrium // HSC Chemistry YouTube Iron Iii Chloride Water Reaction The chemistry of the (etching) reaction. Anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. By dissolving iron ore in hcl (hydrochloric acid) fe 3 o 4 + 8hcl → fecl 2 + 2fecl 3 + 4h 2 o. Preparation of ferric chloride solution. When dissolved in water, it undergoes hydrolysis, resulting in the formation of. Iron Iii Chloride Water Reaction.