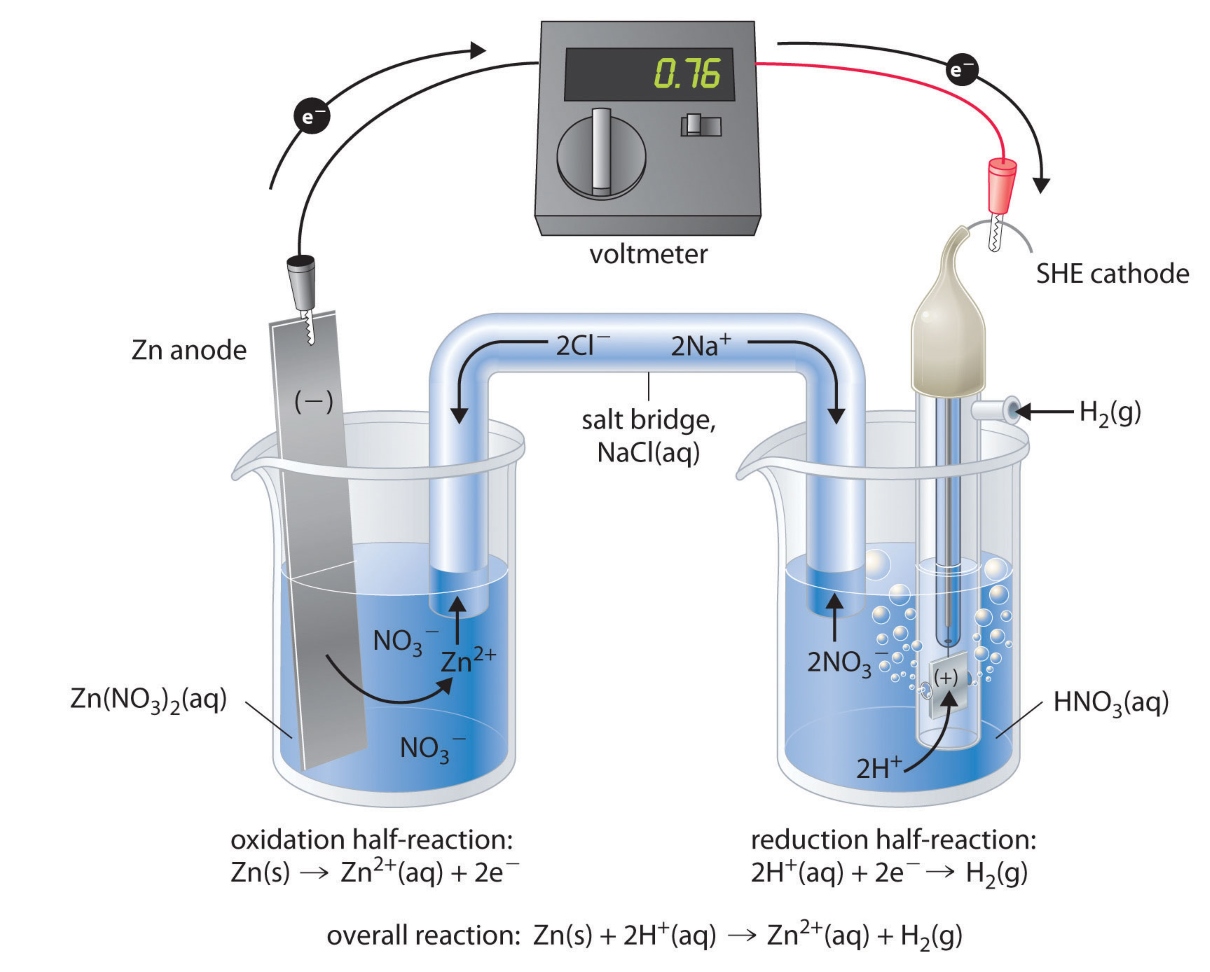
Is A Galvanic Cell Spontaneous . Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. It is also known as a voltaic cell.
from facts.net
A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. Galvanic cells are electrochemical cells that can be used to do work. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. It is also known as a voltaic cell.
9 Enigmatic Facts About Galvanic Cell
Is A Galvanic Cell Spontaneous A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. It is also known as a voltaic cell. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process.
From www.nanoscience.com
Electrochemistry Nanoscience Instruments Is A Galvanic Cell Spontaneous A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to. Is A Galvanic Cell Spontaneous.
From www.slideserve.com
PPT The End is in Site! PowerPoint Presentation, free download ID Is A Galvanic Cell Spontaneous It is also known as a voltaic cell. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic cell based on the spontaneous reaction between. Is A Galvanic Cell Spontaneous.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Is A Galvanic Cell Spontaneous A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell is an electrochemical cell that converts the chemical. Is A Galvanic Cell Spontaneous.
From 2012books.lardbucket.org
Electrochemistry Is A Galvanic Cell Spontaneous A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. It is also known as a voltaic cell. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic. Is A Galvanic Cell Spontaneous.
From glossary.periodni.com
Galvanic Chemistry Dictionary & Glossary Is A Galvanic Cell Spontaneous A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. Galvanic cells are electrochemical cells that can be used to do work. It is also known as a voltaic cell. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell, on the other hand, produces. Is A Galvanic Cell Spontaneous.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Is A Galvanic Cell Spontaneous A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. Galvanic cells are electrochemical cells that can be used to do work. A galvanic. Is A Galvanic Cell Spontaneous.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Is A Galvanic Cell Spontaneous Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells are electrochemical cells that can be used to do work. It is. Is A Galvanic Cell Spontaneous.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Is A Galvanic Cell Spontaneous A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. Galvanic cells are electrochemical cells that can be used to do work. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. It is also known as a voltaic cell. A galvanic (voltaic) cell uses the energy released during. Is A Galvanic Cell Spontaneous.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Is A Galvanic Cell Spontaneous Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous. Is A Galvanic Cell Spontaneous.
From www.youtube.com
How To Draw Galvanic Cells and Voltaic Cells Electrochemistry YouTube Is A Galvanic Cell Spontaneous Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous. Is A Galvanic Cell Spontaneous.
From www.slideserve.com
PPT Electrochemistry Part II The Galvanic Cell PowerPoint Is A Galvanic Cell Spontaneous A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell, on the other hand, produces electrical energy as a result of a. Is A Galvanic Cell Spontaneous.
From www.doubtnut.com
In the galvanic cell shown below, which arrow indicates the spontaneou Is A Galvanic Cell Spontaneous A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. Galvanic cells are electrochemical cells that can be used to do work. A galvanic (voltaic) cell uses the energy released during a spontaneous. Is A Galvanic Cell Spontaneous.
From facts.net
9 Enigmatic Facts About Galvanic Cell Is A Galvanic Cell Spontaneous Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force. Is A Galvanic Cell Spontaneous.
From byjus.com
What is the free energy change for galvanic cell? Is A Galvanic Cell Spontaneous It is also known as a voltaic cell. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted. Is A Galvanic Cell Spontaneous.
From www.slideserve.com
PPT Electrochemistry Part II The Galvanic Cell PowerPoint Is A Galvanic Cell Spontaneous It is also known as a voltaic cell. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic (voltaic). Is A Galvanic Cell Spontaneous.
From stock.adobe.com
A galvanic cell or voltaic cell, is an electrochemical cell in which an Is A Galvanic Cell Spontaneous It is also known as a voltaic cell. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. Galvanic. Is A Galvanic Cell Spontaneous.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Is A Galvanic Cell Spontaneous A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. It is also known as a voltaic cell. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur.. Is A Galvanic Cell Spontaneous.
From scienceinfo.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Is A Galvanic Cell Spontaneous A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. It is also known as a voltaic cell. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell is an electrochemical cell. Is A Galvanic Cell Spontaneous.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Is A Galvanic Cell Spontaneous A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. It is also known as a voltaic cell. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. Galvanic cells. Is A Galvanic Cell Spontaneous.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Is A Galvanic Cell Spontaneous Galvanic cells are electrochemical cells that can be used to do work. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted. Is A Galvanic Cell Spontaneous.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 110 Is A Galvanic Cell Spontaneous A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic (voltaic) cell uses the energy released during a spontaneous redox. Is A Galvanic Cell Spontaneous.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Is A Galvanic Cell Spontaneous A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. It is also known as a voltaic cell. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic (voltaic). Is A Galvanic Cell Spontaneous.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Is A Galvanic Cell Spontaneous A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. It is also known as a voltaic cell. Galvanic cells. Is A Galvanic Cell Spontaneous.
From www.clutchprep.com
Galvanic Cell Chemistry Video Clutch Prep Is A Galvanic Cell Spontaneous A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted. Is A Galvanic Cell Spontaneous.
From askfilo.com
A galvanic cell or a voltaic cell which is named after the eminent scient.. Is A Galvanic Cell Spontaneous A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox. Is A Galvanic Cell Spontaneous.
From courses.lumenlearning.com
Galvanic Cells Chemistry Is A Galvanic Cell Spontaneous It is also known as a voltaic cell. A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions. Is A Galvanic Cell Spontaneous.
From www.alamy.com
Illustration of a voltaic cell, or galvanic cell, an electrochemical Is A Galvanic Cell Spontaneous Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. Galvanic cells are electrochemical cells that can be used to. Is A Galvanic Cell Spontaneous.
From www.collegesearch.in
Galvanic Cells Definitions, Examples, How They are Created, Principles Is A Galvanic Cell Spontaneous A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. A galvanic cell, on the other hand, produces. Is A Galvanic Cell Spontaneous.
From ch302.cm.utexas.edu
electrolytic cell Is A Galvanic Cell Spontaneous Galvanic cells are electrochemical cells that can be used to do work. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox. Is A Galvanic Cell Spontaneous.
From pediaa.com
Difference Between Daniell Cell and Galvanic Cell Definition, How Is A Galvanic Cell Spontaneous A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. Galvanic cells are electrochemical cells that can be used. Is A Galvanic Cell Spontaneous.
From www.pinterest.com
Galvanic Cell Animation Galvanic cell, Cell, Electrical energy Is A Galvanic Cell Spontaneous A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. It is also known as a voltaic cell. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A. Is A Galvanic Cell Spontaneous.
From studylib.net
The redox reaction in a galvanic cell is a spontaneous reaction. For Is A Galvanic Cell Spontaneous A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic cell is an electrochemical cell that converts. Is A Galvanic Cell Spontaneous.
From mrselliott.wordpress.com
Electrochemistry, featuring electrolysis and fuel cells. mrs elliott Is A Galvanic Cell Spontaneous A galvanic cell, on the other hand, produces electrical energy as a result of a spontaneous redox process. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. A galvanic cell is an electrochemical cell that converts. Is A Galvanic Cell Spontaneous.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download Is A Galvanic Cell Spontaneous Figure 19.3.3 shows a typical galvanic cell that uses the spontaneous (zn +2. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to. Is A Galvanic Cell Spontaneous.
From www.alamy.com
A galvanic cell or voltaic cell is an electrochemical cell that derives Is A Galvanic Cell Spontaneous Galvanic cells are electrochemical cells that can be used to do work. A galvanic cell based on the spontaneous reaction between copper and silver (i) is depicted in figure 17.3. A galvanic cell is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. A galvanic cell, on the other hand, produces electrical energy as. Is A Galvanic Cell Spontaneous.