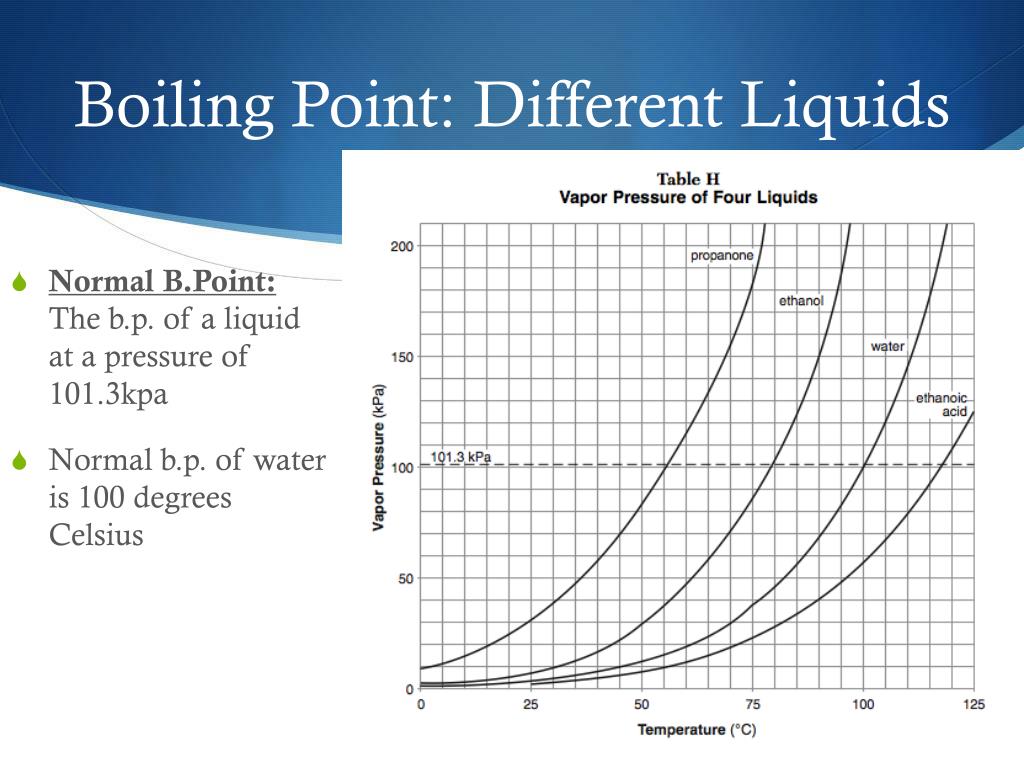
What Happens To Water At Boiling Point . The boiling point of a liquid varies according to the applied pressure; Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. Another key factor affecting boiling point is purity. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. If you drop the pressure to a partial vacuum, water boils at room temperature. The boiling point of a liquid depends on temperature, atmospheric pressure,. At sea level, water boils at 100° c (212° f). Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. At higher altitudes the temperature of the boiling point is lower. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure.
from mavink.com
The boiling point of a liquid varies according to the applied pressure; For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The boiling point of a liquid depends on temperature, atmospheric pressure,. Another key factor affecting boiling point is purity. If you drop the pressure to a partial vacuum, water boils at room temperature. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. At higher altitudes the temperature of the boiling point is lower. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again.
Water Boiling Point Pressure Chart
What Happens To Water At Boiling Point Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. If you drop the pressure to a partial vacuum, water boils at room temperature. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of a liquid depends on temperature, atmospheric pressure,. Another key factor affecting boiling point is purity. The boiling point of a liquid varies according to the applied pressure; At sea level, water boils at 100° c (212° f). Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. At higher altitudes the temperature of the boiling point is lower. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Happens To Water At Boiling Point If you drop the pressure to a partial vacuum, water boils at room temperature. The boiling point of a liquid depends on temperature, atmospheric pressure,. Another key factor affecting boiling point is purity. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. At higher altitudes the temperature of the boiling point is lower.. What Happens To Water At Boiling Point.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Happens To Water At Boiling Point The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Another key factor affecting boiling point is purity. The boiling point of a liquid depends on temperature, atmospheric pressure,. At higher altitudes the temperature of the boiling point is. What Happens To Water At Boiling Point.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) What Happens To Water At Boiling Point Another key factor affecting boiling point is purity. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. The boiling point of a liquid varies according to the applied pressure; The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Boiling of a pure substance. What Happens To Water At Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart What Happens To Water At Boiling Point Another key factor affecting boiling point is purity. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. The boiling point of a liquid varies according to the applied pressure; At higher altitudes. What Happens To Water At Boiling Point.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For What Happens To Water At Boiling Point The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The boiling point of a liquid depends on temperature, atmospheric pressure,. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Another key factor affecting boiling point is purity. Because atmospheric pressure can change. What Happens To Water At Boiling Point.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting What Happens To Water At Boiling Point The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The boiling point of a liquid varies according to the applied pressure; The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. At higher altitudes the temperature of the boiling point is lower. For. What Happens To Water At Boiling Point.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Happens To Water At Boiling Point Another key factor affecting boiling point is purity. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. At sea level, water boils at 100° c (212° f). The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The boiling point of a liquid. What Happens To Water At Boiling Point.
From www.youtube.com
How To Draw Determination Of Boiling Point Of Water Diagram Easily Step What Happens To Water At Boiling Point The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. The boiling point of a liquid varies according to the applied pressure; Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. Another key factor affecting boiling point is purity. Note that if cooling had been applied. What Happens To Water At Boiling Point.
From www.animalia-life.club
Boiling Point Of Water For Kids What Happens To Water At Boiling Point The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The lower vapor pressure corresponds to. What Happens To Water At Boiling Point.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Happens To Water At Boiling Point At sea level, water boils at 100° c (212° f). At higher altitudes the temperature of the boiling point is lower. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The boiling point of a liquid varies according to the applied pressure; For example, for water, the boiling point is. What Happens To Water At Boiling Point.
From www.slideserve.com
PPT What Happens when W ater Boils? PowerPoint Presentation, free What Happens To Water At Boiling Point At sea level, water boils at 100° c (212° f). The boiling point of a liquid varies according to the applied pressure; At higher altitudes the temperature of the boiling point is lower. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. If you drop the pressure to a partial vacuum, water. What Happens To Water At Boiling Point.
From www.youtube.com
Measurement of boiling point of water practically YouTube What Happens To Water At Boiling Point The boiling point of a liquid varies according to the applied pressure; At higher altitudes the temperature of the boiling point is lower. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. If you drop the pressure. What Happens To Water At Boiling Point.
From www.worksheetsplanet.com
What is Boiling Point What Happens To Water At Boiling Point If you drop the pressure to a partial vacuum, water boils at room temperature. At higher altitudes the temperature of the boiling point is lower. Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The. What Happens To Water At Boiling Point.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For What Happens To Water At Boiling Point For example, for water, the boiling point is 100ºc at a pressure of 1 atm. If you drop the pressure to a partial vacuum, water boils at room temperature. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. The lower vapor pressure corresponds to a lower boiling point, and therefore. What Happens To Water At Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Happens To Water At Boiling Point At higher altitudes the temperature of the boiling point is lower. Another key factor affecting boiling point is purity. The boiling point of a liquid varies according to the applied pressure; The boiling point of a liquid depends on temperature, atmospheric pressure,. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to. What Happens To Water At Boiling Point.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Happens To Water At Boiling Point At sea level, water boils at 100° c (212° f). Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external. What Happens To Water At Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Happens To Water At Boiling Point Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. At sea level, water. What Happens To Water At Boiling Point.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Happens To Water At Boiling Point At sea level, water boils at 100° c (212° f). The boiling point of a liquid depends on temperature, atmospheric pressure,. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. At higher altitudes the temperature of the boiling point is lower. If you drop the pressure to a partial vacuum, water boils. What Happens To Water At Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Happens To Water At Boiling Point The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. If you drop the pressure. What Happens To Water At Boiling Point.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Happens To Water At Boiling Point The boiling point of a liquid varies according to the applied pressure; Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. The boiling point of a liquid depends on temperature, atmospheric pressure,. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure.. What Happens To Water At Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Happens To Water At Boiling Point At sea level, water boils at 100° c (212° f). Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external. What Happens To Water At Boiling Point.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Happens To Water At Boiling Point If you drop the pressure to a partial vacuum, water boils at room temperature. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Another key factor affecting boiling point is purity. The normal boiling point is. What Happens To Water At Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Happens To Water At Boiling Point At higher altitudes the temperature of the boiling point is lower. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. At sea level, water boils at 100° c (212° f). The boiling point of a liquid. What Happens To Water At Boiling Point.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Happens To Water At Boiling Point At sea level, water boils at 100° c (212° f). Another key factor affecting boiling point is purity. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of a liquid varies according to the applied pressure; Note that if cooling had been applied to the liquid on the bottom, these subsequent. What Happens To Water At Boiling Point.
From www.youtube.com
Boiling Point of Water (H2O) YouTube What Happens To Water At Boiling Point Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of a liquid varies according. What Happens To Water At Boiling Point.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country What Happens To Water At Boiling Point The boiling point of a liquid depends on temperature, atmospheric pressure,. The boiling point of a liquid varies according to the applied pressure; Another key factor affecting boiling point is purity. At sea level, water boils at 100° c (212° f). For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The lower vapor pressure. What Happens To Water At Boiling Point.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Happens To Water At Boiling Point The boiling point of a liquid depends on temperature, atmospheric pressure,. At higher altitudes the temperature of the boiling point is lower. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. At sea level, water boils at 100° c (212° f). Note that if cooling had been applied to the liquid on the bottom,. What Happens To Water At Boiling Point.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID1624745 What Happens To Water At Boiling Point At sea level, water boils at 100° c (212° f). If you drop the pressure to a partial vacuum, water boils at room temperature. At higher altitudes the temperature of the boiling point is lower. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. The boiling point of a liquid. What Happens To Water At Boiling Point.
From www.animalia-life.club
Boiling Point Of Water Examples What Happens To Water At Boiling Point At sea level, water boils at 100° c (212° f). The boiling point of a liquid depends on temperature, atmospheric pressure,. For example, for water, the boiling point is 100ºc at a pressure of 1 atm. The boiling point of a liquid varies according to the applied pressure; Because atmospheric pressure can change based on location, the boiling point of. What Happens To Water At Boiling Point.
From howchimp.com
What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit What Happens To Water At Boiling Point If you drop the pressure to a partial vacuum, water boils at room temperature. At sea level, water boils at 100° c (212° f). Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal. What Happens To Water At Boiling Point.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Happens To Water At Boiling Point Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. The boiling point of a liquid depends on temperature, atmospheric pressure,. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. For example, for water, the boiling point is 100ºc at a pressure of 1. What Happens To Water At Boiling Point.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free What Happens To Water At Boiling Point Another key factor affecting boiling point is purity. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. The boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. Note that if cooling. What Happens To Water At Boiling Point.
From lyndajguilleno.blob.core.windows.net
What Happens To Water When It Reaches Its Boiling Point at What Happens To Water At Boiling Point The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. If you drop the pressure to a partial vacuum, water boils at room temperature. The lower vapor pressure corresponds to a lower boiling point, and therefore the water boils again. For example, for water, the boiling point is 100ºc at. What Happens To Water At Boiling Point.
From lyndajguilleno.blob.core.windows.net
What Happens To Water When It Reaches Its Boiling Point at What Happens To Water At Boiling Point Note that if cooling had been applied to the liquid on the bottom, these subsequent boilings would not occur. Boiling of a pure substance occurs at a particular constant temperature called boiling point or boiling temperature. The boiling point of a liquid varies according to the applied pressure; At higher altitudes the temperature of the boiling point is lower. Another. What Happens To Water At Boiling Point.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Happens To Water At Boiling Point The boiling point of a liquid varies according to the applied pressure; If you drop the pressure to a partial vacuum, water boils at room temperature. Because atmospheric pressure can change based on location, the boiling point of a liquid changes with the external pressure. Boiling of a pure substance occurs at a particular constant temperature called boiling point or. What Happens To Water At Boiling Point.