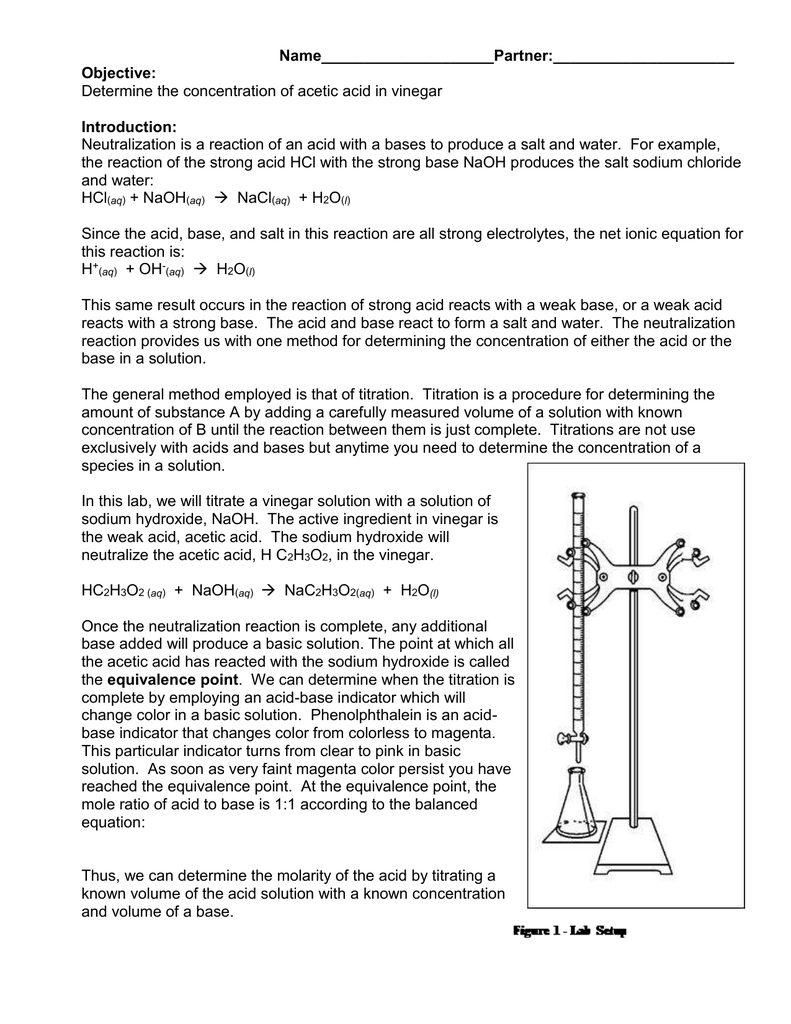
Titration Lab Questions . At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. The change you observe is actually from a tiny bit of extra titrant; Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. Titration level 1 titration level 2 titration level 3 titration level 4. Keeping the extra as small as possible will give you the most accurate. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Always place a container such as a small beaker below the buret when you are filling it. This resource has been developed in.
from mungfali.com
Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. Always place a container such as a small beaker below the buret when you are filling it. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Titration level 1 titration level 2 titration level 3 titration level 4. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Keeping the extra as small as possible will give you the most accurate. This resource has been developed in. The change you observe is actually from a tiny bit of extra titrant;
Titration Lab Diagram
Titration Lab Questions Titration level 1 titration level 2 titration level 3 titration level 4. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. The change you observe is actually from a tiny bit of extra titrant; Keeping the extra as small as possible will give you the most accurate. Titration level 1 titration level 2 titration level 3 titration level 4. Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. Always place a container such as a small beaker below the buret when you are filling it. This resource has been developed in.
From mungfali.com
Titration Lab Diagram Titration Lab Questions Titration level 1 titration level 2 titration level 3 titration level 4. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration. Titration Lab Questions.
From www.youtube.com
Answering a standard REDOX titration question YouTube Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. Titration level 1 titration level 2 titration level. Titration Lab Questions.
From exovevmgr.blob.core.windows.net
Titration Lab Quiz at Roger Owen blog Titration Lab Questions The change you observe is actually from a tiny bit of extra titrant; Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Lab day #2) use the successful trials to calculate an average volume of naoh used to. Titration Lab Questions.
From www.studypool.com
SOLUTION Week base strong acid titration lab report and post lab Titration Lab Questions This resource has been developed in. Always place a container such as a small beaker below the buret when you are filling it. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Lab day #2) use the successful. Titration Lab Questions.
From www.chegg.com
Solved PART B Acid Base Titration Lab Report I Titration Lab Questions Titration level 1 titration level 2 titration level 3 titration level 4. The change you observe is actually from a tiny bit of extra titrant; Always place a container such as a small beaker below the buret when you are filling it. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate,. Titration Lab Questions.
From mungfali.com
Titration Steps Titration Lab Questions The change you observe is actually from a tiny bit of extra titrant; Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the. Titration Lab Questions.
From www.chegg.com
Solved AcidBase Titration Virtual Lab Introduction In Titration Lab Questions Titration level 1 titration level 2 titration level 3 titration level 4. Keeping the extra as small as possible will give you the most accurate. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. The change you observe is actually from a tiny bit. Titration Lab Questions.
From www.studocu.com
Titration Lab Sheet What does this tell you about the substance? it’s Titration Lab Questions The change you observe is actually from a tiny bit of extra titrant; This resource has been developed in. Always place a container such as a small beaker below the buret when you are filling it. Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the. Titration Lab Questions.
From www.chegg.com
Solved Lab 6 Titrations PostLab Assignment Each student Titration Lab Questions Always place a container such as a small beaker below the buret when you are filling it. The change you observe is actually from a tiny bit of extra titrant; This resource has been developed in. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by. Titration Lab Questions.
From www.youtube.com
Titration Lab, part 2, old version of a question YouTube Titration Lab Questions Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Titration level 1 titration level 2 titration level 3 titration level 4. The change you observe is actually from a tiny bit of extra titrant; Keeping the extra as. Titration Lab Questions.
From www.chegg.com
Solved Titration Curves Worksheet The following are Weak Titration Lab Questions Keeping the extra as small as possible will give you the most accurate. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. The change you observe is actually from a tiny bit of extra titrant; This resource has been developed in. Always place a. Titration Lab Questions.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Titration Lab Questions Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. At the. Titration Lab Questions.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 ans 93ghs Studypool Titration Lab Questions Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. This resource has been developed in. The change you observe is actually from a tiny bit of extra titrant; Always place a container such as a small beaker below the buret when you. Titration Lab Questions.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Titration Lab Questions Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Keeping the extra as small as possible will give you the most accurate. The change you observe is actually from a tiny bit of extra titrant; Lab day #2). Titration Lab Questions.
From armsingle10.pythonanywhere.com
Breathtaking Titration Of Khp With Naoh Calculations Balancing Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. The change you observe. Titration Lab Questions.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Titration Lab Questions Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. This resource has been developed in. Keeping the extra as small as possible will give you the most accurate. The change you observe is actually from a tiny bit. Titration Lab Questions.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Titration Lab Questions The change you observe is actually from a tiny bit of extra titrant; Titration level 1 titration level 2 titration level 3 titration level 4. Always place a container such as a small beaker below the buret when you are filling it. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate,. Titration Lab Questions.
From studylib.net
Titration lab Titration Lab Questions Keeping the extra as small as possible will give you the most accurate. This resource has been developed in. The change you observe is actually from a tiny bit of extra titrant; Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the. Titration Lab Questions.
From klajqxgeq.blob.core.windows.net
Titration Question Examples at Larry Magno blog Titration Lab Questions Titration level 1 titration level 2 titration level 3 titration level 4. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Keeping the extra as small as possible will give you the most accurate. At the equivalence point. Titration Lab Questions.
From mungfali.com
Titration Lab Diagram Titration Lab Questions Always place a container such as a small beaker below the buret when you are filling it. Keeping the extra as small as possible will give you the most accurate. Titration level 1 titration level 2 titration level 3 titration level 4. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of. Titration Lab Questions.
From www.studocu.com
Titration questions and answers 1. Titration (Higher Level) 2002 Titration Lab Questions The change you observe is actually from a tiny bit of extra titrant; At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Keeping the extra as small as possible will give you the most accurate. Always place a container such as a small beaker. Titration Lab Questions.
From www.studypool.com
SOLUTION Answer To Titration Lab Questions Studypool Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. Titration level 1 titration. Titration Lab Questions.
From mavink.com
Acid Base Titration Lab Procedure Titration Lab Questions Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. This resource has been developed in. The change you observe is actually from a tiny bit of extra titrant; Keeping the extra as small as possible will give you. Titration Lab Questions.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Titration level 1 titration level 2 titration level 3 titration level 4. Keeping the extra as small as possible will give you the most accurate. This resource has been developed in. Lab day #2) use. Titration Lab Questions.
From ceplknbb.blob.core.windows.net
Acid Base Titration Questions at Deborah Wedel blog Titration Lab Questions This resource has been developed in. Always place a container such as a small beaker below the buret when you are filling it. Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. Titration is a process of measuring the concentration of an. Titration Lab Questions.
From www.chegg.com
Solved LAB 7 An Introduction to Titrations 63 Questions 1. Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. This resource has been developed in. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two. Titration Lab Questions.
From studylib.net
Acid/Base Titration Lab Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Keeping the extra as small as possible will give you the most accurate. Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to. Titration Lab Questions.
From materialmediamoench.z19.web.core.windows.net
Titration Lab Answer Key Titration Lab Questions Titration level 1 titration level 2 titration level 3 titration level 4. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Always place a container such as a small beaker below the buret when you are filling it. Keeping the extra as small as. Titration Lab Questions.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titration Lab Questions Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two is complete. The change you observe is actually from a tiny bit of extra titrant; This resource has been developed in. Titration level 1 titration level 2 titration level 3 titration. Titration Lab Questions.
From www.chemistryworksheet.com
Chemistry Titration Worksheet Answers Titration Lab Questions Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. Always place a container such as a small beaker below the buret when you are filling it. Keeping the extra as small as possible will give you the most accurate. This resource has. Titration Lab Questions.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Titration level 1 titration level 2 titration level 3 titration level 4. Always place a container such as a small beaker below the buret when you are filling it. This resource has been developed in.. Titration Lab Questions.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. This resource has been developed in. Titration is a process of measuring the concentration of an unknown solution by adding a known amount of another solution of known concentration until the reaction between the two. Titration Lab Questions.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Titration Lab Questions At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Always place a container such as a small beaker below the buret when you are filling it. Keeping the extra as small as possible will give you the most accurate. This resource has been developed. Titration Lab Questions.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Lab Questions The change you observe is actually from a tiny bit of extra titrant; Titration level 1 titration level 2 titration level 3 titration level 4. At the equivalence point all the acetic acid has been neutralized and converted to its conjugate base acetate, so the ph is determined by the. Keeping the extra as small as possible will give you. Titration Lab Questions.
From www.youtube.com
TITRATION EXAM QUESTION for A LEVEL CHEMISTRY YouTube Titration Lab Questions Keeping the extra as small as possible will give you the most accurate. Titration level 1 titration level 2 titration level 3 titration level 4. Lab day #2) use the successful trials to calculate an average volume of naoh used to titrate the unknown acid, and to calculate the concentration of the. Always place a container such as a small. Titration Lab Questions.