Bromine Has How Many Electrons . The atomic number for bromine is 35, which means it has 35 protons in its atomic. These electrons are arranged in various energy. It was the first element to be extracted. The br− ion has 36 electrons. Bromine has only seven electrons in its last orbit. Bromine has an atomic number of 35, which means that its atom. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Therefore, the number of electrons in neutral atom of bromine is 35. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. It has an atomic weight of 79.904 and a mass number of.
from www.animalia-life.club
The br− ion has 36 electrons. Bromine has an atomic number of 35, which means that its atom. Therefore, the number of electrons in neutral atom of bromine is 35. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It was the first element to be extracted. It has an atomic weight of 79.904 and a mass number of. Bromine is the 35th element in the periodic table and the symbol is ‘br’. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. The atomic number for bromine is 35, which means it has 35 protons in its atomic. Bromine has only seven electrons in its last orbit.
Electron Configuration For Bromine
Bromine Has How Many Electrons The atomic number for bromine is 35, which means it has 35 protons in its atomic. It has an atomic weight of 79.904 and a mass number of. It was the first element to be extracted. Therefore, the number of electrons in neutral atom of bromine is 35. The br− ion has 36 electrons. Bromine is the 35th element in the periodic table and the symbol is ‘br’. The atomic number for bromine is 35, which means it has 35 protons in its atomic. Bromine has an atomic number of 35, which means that its atom. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. These electrons are arranged in various energy. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine has only seven electrons in its last orbit. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has How Many Electrons Bromine is the 35th element in the periodic table and the symbol is ‘br’. The br− ion has 36 electrons. The atomic number for bromine is 35, which means it has 35 protons in its atomic. It was the first element to be extracted. In an atomic ion only the number of electrons changes but the number of protons and. Bromine Has How Many Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has How Many Electrons The atomic number for bromine is 35, which means it has 35 protons in its atomic. Therefore, the number of electrons in neutral atom of bromine is 35. Bromine has only seven electrons in its last orbit. Bromine has an atomic number of 35, which means that its atom. The br− ion has 36 electrons. Bromine, with its atomic number. Bromine Has How Many Electrons.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Has How Many Electrons Bromine is the 35th element in the periodic table and the symbol is ‘br’. The br− ion has 36 electrons. Bromine has only seven electrons in its last orbit. It was the first element to be extracted. Therefore, the number of electrons in neutral atom of bromine is 35. Bromine has an atomic number of 35, which means that its. Bromine Has How Many Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Has How Many Electrons It has an atomic weight of 79.904 and a mass number of. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine has only seven electrons in its last orbit. It was the first element to be extracted. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. These electrons. Bromine Has How Many Electrons.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine Has How Many Electrons It was the first element to be extracted. Bromine is the 35th element in the periodic table and the symbol is ‘br’. The br− ion has 36 electrons. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. Bromine has an atomic number of 35, which means that its atom.. Bromine Has How Many Electrons.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Has How Many Electrons It has an atomic weight of 79.904 and a mass number of. The atomic number for bromine is 35, which means it has 35 protons in its atomic. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. The br− ion has 36 electrons. Bromine has only seven electrons in. Bromine Has How Many Electrons.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare Bromine Has How Many Electrons It was the first element to be extracted. Bromine has an atomic number of 35, which means that its atom. Therefore, the number of electrons in neutral atom of bromine is 35. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. These electrons are arranged in various energy. Bromine is the 35th element in. Bromine Has How Many Electrons.
From www.numerade.com
SOLVED Number 7 (a) Identify the number of electrons in the ground Bromine Has How Many Electrons Bromine has an atomic number of 35, which means that its atom. The br− ion has 36 electrons. The atomic number for bromine is 35, which means it has 35 protons in its atomic. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Therefore, the number of electrons in neutral atom of bromine is 35.. Bromine Has How Many Electrons.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in Bromine Has How Many Electrons Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. The br− ion has 36 electrons. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of. In an atomic ion only the number. Bromine Has How Many Electrons.
From www.dreamstime.com
Bromine stock illustration. Illustration of render, formula 139651101 Bromine Has How Many Electrons Therefore, the number of electrons in neutral atom of bromine is 35. Bromine has an atomic number of 35, which means that its atom. The br− ion has 36 electrons. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine has only seven electrons in its last orbit. In an atomic ion only the number. Bromine Has How Many Electrons.
From www.dreamstime.com
Atom of Bromine with Detailed Core and Its 35 Electrons on Black Stock Bromine Has How Many Electrons It was the first element to be extracted. The br− ion has 36 electrons. These electrons are arranged in various energy. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. It has an atomic weight of 79.904 and a mass number of. The atomic number for bromine is 35,. Bromine Has How Many Electrons.
From www.gauthmath.com
Solved How many protons, electron and neutron does bromine ion have Bromine Has How Many Electrons Bromine has an atomic number of 35, which means that its atom. The br− ion has 36 electrons. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. The atomic number for bromine is 35, which means it has 35 protons in its atomic. It has an atomic weight of 79.904 and a mass number. Bromine Has How Many Electrons.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and Bromine Has How Many Electrons In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. Bromine has only seven electrons in its last orbit. Therefore, the number of electrons in neutral atom of bromine is 35. It was the first. Bromine Has How Many Electrons.
From www.numerade.com
SOLVED How many electrons are around bromine 341 Lewis structure of Bromine Has How Many Electrons The br− ion has 36 electrons. Bromine is the 35th element in the periodic table and the symbol is ‘br’. These electrons are arranged in various energy. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The atomic number for bromine is 35, which means it has 35 protons. Bromine Has How Many Electrons.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Bromine Has How Many Electrons It has an atomic weight of 79.904 and a mass number of. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. The br− ion has 36 electrons. These electrons are arranged in various energy. The atomic number for bromine is 35, which means it has 35 protons in its. Bromine Has How Many Electrons.
From exooeohnk.blob.core.windows.net
Bromine Total Number Of Valence Electrons at Rebeca Henshaw blog Bromine Has How Many Electrons Bromine has an atomic number of 35, which means that its atom. Bromine has only seven electrons in its last orbit. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The br− ion has 36 electrons. It has an atomic weight of 79.904 and a mass number of. In. Bromine Has How Many Electrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Has How Many Electrons Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. Bromine has an atomic number of 35, which means that its atom. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. Bromine is the 35th element in the periodic table and the symbol is. Bromine Has How Many Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Has How Many Electrons It has an atomic weight of 79.904 and a mass number of. It was the first element to be extracted. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. Bromine has an atomic number of 35, which means that its atom. The atomic number for bromine is 35, which. Bromine Has How Many Electrons.
From ar.inspiredpencil.com
Electron Configuration Bromine Bromine Has How Many Electrons In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. The atomic number for bromine is 35, which means it has 35 protons in its atomic. Bromine is the 35th element in the periodic table and the symbol is ‘br’. The br− ion has 36 electrons. Therefore, the number of. Bromine Has How Many Electrons.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Bromine Has How Many Electrons The br− ion has 36 electrons. Bromine is the 35th element in the periodic table and the symbol is ‘br’. It has an atomic weight of 79.904 and a mass number of. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. These electrons are arranged in various energy. In. Bromine Has How Many Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Has How Many Electrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. Bromine has an atomic number of 35, which means that its atom. It has an atomic weight of 79.904 and a mass number of. The. Bromine Has How Many Electrons.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Bromine Has How Many Electrons In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. It was the first element to be extracted. Therefore, the number of electrons in neutral atom of bromine is 35. It has an atomic weight of 79.904 and a mass number of. These electrons are arranged in various energy. Bromine. Bromine Has How Many Electrons.
From www.slideserve.com
PPT Atoms & Chemical Bonding PowerPoint Presentation, free download Bromine Has How Many Electrons Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. Bromine is the 35th element in the periodic table and the symbol is ‘br’. It has an atomic weight of 79.904 and a mass number of. The br− ion has 36 electrons. Therefore, the number of electrons in neutral atom of bromine is 35. These. Bromine Has How Many Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has How Many Electrons The br− ion has 36 electrons. The atomic number for bromine is 35, which means it has 35 protons in its atomic. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. Bromine has an atomic number of 35, which means that its atom. Bromine is the 35th element in the periodic table and the. Bromine Has How Many Electrons.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) Bromine Has How Many Electrons In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine has only seven electrons in its last orbit. It was. Bromine Has How Many Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has How Many Electrons Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. It has an atomic weight of 79.904 and a mass number of. Bromine is the 35th element in the periodic table and the symbol is ‘br’. It was the first element to be extracted. The br− ion has 36 electrons. Bromine has only seven electrons. Bromine Has How Many Electrons.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Has How Many Electrons These electrons are arranged in various energy. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. It was the first element to be extracted. The br− ion has 36 electrons. Therefore, the number of electrons in neutral atom of bromine is 35. Bromine, with its atomic number 35, has. Bromine Has How Many Electrons.
From www.slideshare.net
Bromine berry Bromine Has How Many Electrons The br− ion has 36 electrons. Bromine is the 35th element in the periodic table and the symbol is ‘br’. The atomic number for bromine is 35, which means it has 35 protons in its atomic. It has an atomic weight of 79.904 and a mass number of. Bromine has an atomic number of 35, which means that its atom.. Bromine Has How Many Electrons.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine Has How Many Electrons Bromine is the 35th element in the periodic table and the symbol is ‘br’. It has an atomic weight of 79.904 and a mass number of. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine has only seven electrons in its last orbit. The br− ion has 36. Bromine Has How Many Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Has How Many Electrons Therefore, the number of electrons in neutral atom of bromine is 35. The br− ion has 36 electrons. The atomic number for bromine is 35, which means it has 35 protons in its atomic. Bromine has only seven electrons in its last orbit. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine has an. Bromine Has How Many Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Has How Many Electrons These electrons are arranged in various energy. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine has an atomic number of 35, which means that its atom. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine, with its atomic number 35, has. Bromine Has How Many Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Has How Many Electrons It was the first element to be extracted. The br− ion has 36 electrons. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. Bromine is the 35th element in the periodic table and has a symbol of br and atomic. Bromine Has How Many Electrons.
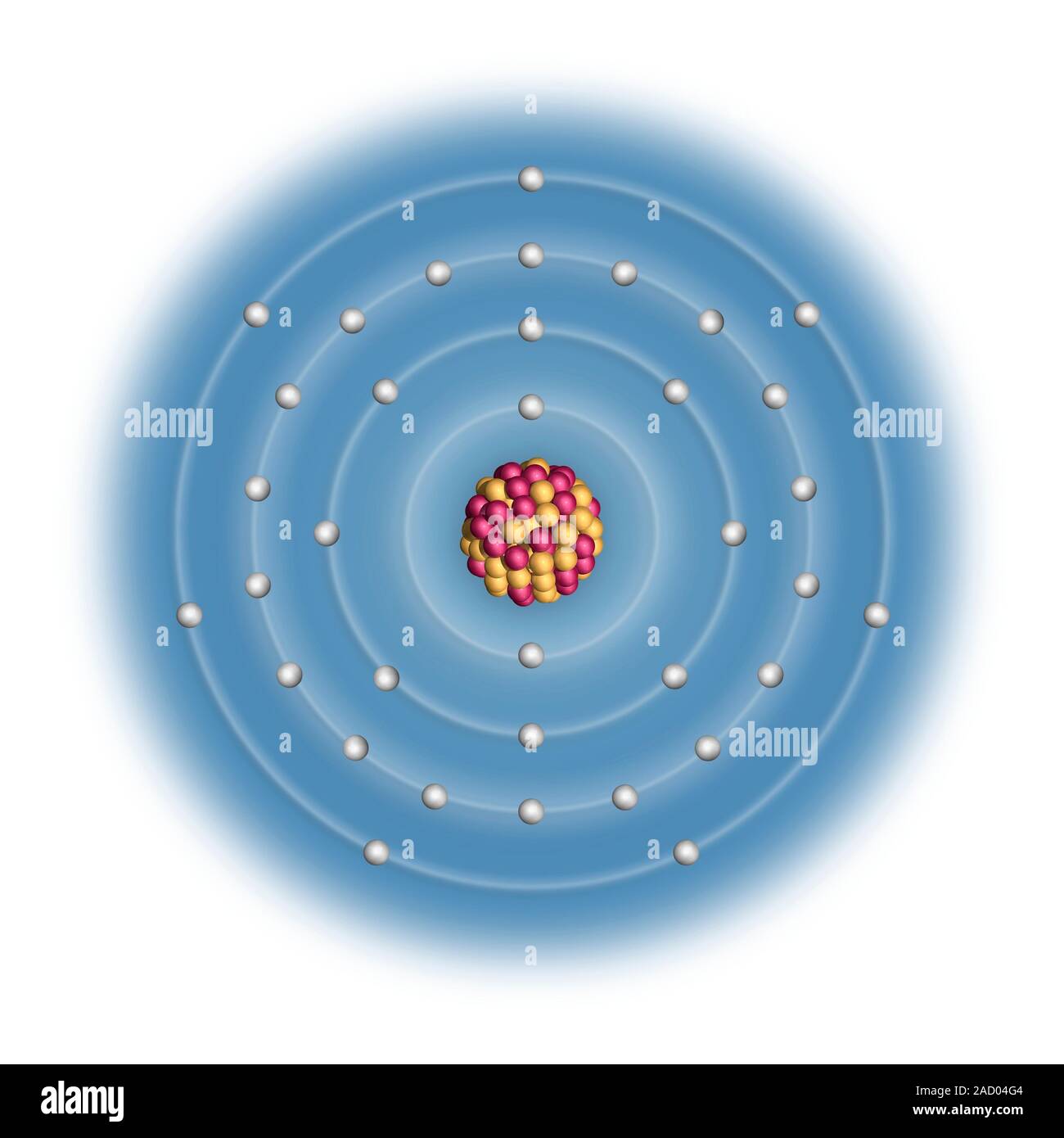
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Has How Many Electrons Bromine has only seven electrons in its last orbit. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. Bromine has an atomic number of 35, which means that its atom. Therefore, the number of electrons in neutral atom of bromine is 35. Bromine is the 35th element in the periodic table and has a. Bromine Has How Many Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Has How Many Electrons Bromine has an atomic number of 35, which means that its atom. The atomic number for bromine is 35, which means it has 35 protons in its atomic. Bromine, with its atomic number 35, has a total of 35 electrons orbiting its nucleus. It was the first element to be extracted. Bromine has only seven electrons in its last orbit.. Bromine Has How Many Electrons.
From slideplayer.com
Development of the Modern Periodic Table ppt download Bromine Has How Many Electrons It was the first element to be extracted. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number. Bromine Has How Many Electrons.