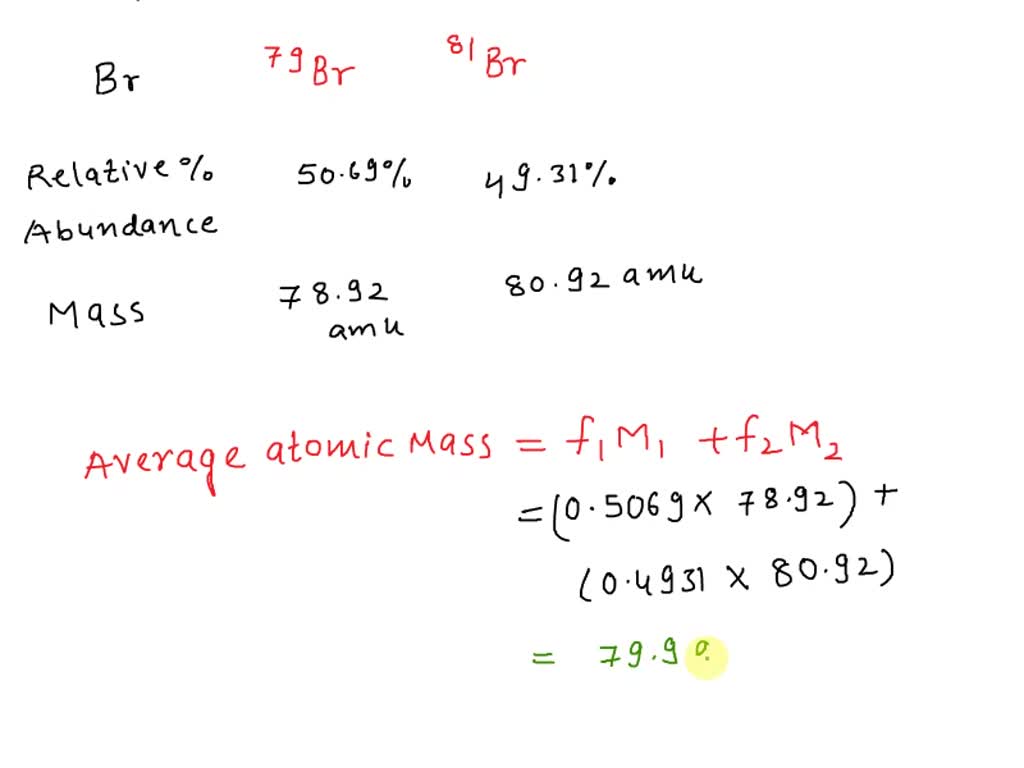Relative Atomic Mass Of Bromine Is 79.9 . How many neutrons are there in 81 br and which isotope is more abundant? Bromine has a relative atomic mass of 79.9 : Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The atomic mass is the mass of an atom. The relative atomic mass of bromine is 79.9. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: 79 br and 81 br. Atomic mass of bromine is 79.904 u. The atomic mass or relative isotopic mass refers to. The relative atomic mass of bromine is 79.9. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following.
from www.numerade.com
If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. The relative atomic mass of bromine is 79.9. The relative atomic mass of bromine is 79.9. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The atomic mass is the mass of an atom. 79 br and 81 br. The atomic mass or relative isotopic mass refers to. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: Atomic mass of bromine is 79.904 u. Bromine has a relative atomic mass of 79.9 :
SOLVED Using the periodic table, What is the average atomic mass of
Relative Atomic Mass Of Bromine Is 79.9 The atomic mass or relative isotopic mass refers to. The atomic mass or relative isotopic mass refers to. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The relative atomic mass of bromine is 79.9. How many neutrons are there in 81 br and which isotope is more abundant? The atomic mass is the mass of an atom. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: Bromine has a relative atomic mass of 79.9 : If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. Atomic mass of bromine is 79.904 u. 79 br and 81 br. The relative atomic mass of bromine is 79.9.
From www.numerade.com
SOLVED Using the periodic table, What is the average atomic mass of Relative Atomic Mass Of Bromine Is 79.9 Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: Atomic mass of bromine is 79.904 u. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. Bromine has a relative atomic mass of 79.9 : The relative atomic mass of bromine is 79.9. The atomic mass or relative isotopic. Relative Atomic Mass Of Bromine Is 79.9.
From www.numerade.com
SOLVED If the relative atomic mass of a bromine atom is 80 u Relative Atomic Mass Of Bromine Is 79.9 The atomic mass or relative isotopic mass refers to. The relative atomic mass of bromine is 79.9. The relative atomic mass of bromine is 79.9. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: Bromine has a relative atomic mass of 79.9 : If you were given the % of each isotope and asked. Relative Atomic Mass Of Bromine Is 79.9.
From www.gauthmath.com
Solved 22 Naturallyoccurring bromine has a relative atomic mass of 80 Relative Atomic Mass Of Bromine Is 79.9 The relative atomic mass of bromine is 79.9. How many neutrons are there in 81 br and which isotope is more abundant? Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. Bromine has a relative atomic mass of 79.9 : The relative atomic mass of bromine is 79.9. The atomic mass is the mass. Relative Atomic Mass Of Bromine Is 79.9.
From www.youtube.com
What is Relative Atomic Mass? Chemical Formula and Equation YouTube Relative Atomic Mass Of Bromine Is 79.9 If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. The relative atomic mass of bromine is 79.9. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: The atomic mass is the mass of an atom. The atomic mass or relative isotopic. Relative Atomic Mass Of Bromine Is 79.9.
From brainly.in
10. If bromine atom is available in the form of, say, two isotopesBr Relative Atomic Mass Of Bromine Is 79.9 Bromine has a relative atomic mass of 79.9 : The relative atomic mass of bromine is 79.9. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: The atomic mass is the mass of an atom. Atomic mass of bromine is 79.904 u. How many neutrons are there in 81 br and which isotope is. Relative Atomic Mass Of Bromine Is 79.9.
From www.slideserve.com
PPT 1 Looking Inside the Black Box PowerPoint Presentation, free Relative Atomic Mass Of Bromine Is 79.9 Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to. The relative atomic mass of bromine is 79.9. Bromine has a relative atomic mass of 79.9 : Bromine has two naturally occurring isotopes and has an average. Relative Atomic Mass Of Bromine Is 79.9.
From loeuqmdmn.blob.core.windows.net
Atomic Mass Of Bromine Class 9 at James Stevens blog Relative Atomic Mass Of Bromine Is 79.9 Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The atomic mass is the mass of an atom. Atomic mass of bromine is 79.904 u. The relative atomic mass of bromine is 79.9. Bromine has a relative atomic mass of 79.9 : If you were given the % of each isotope and asked to. Relative Atomic Mass Of Bromine Is 79.9.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Relative Atomic Mass Of Bromine Is 79.9 The relative atomic mass of bromine is 79.9. Bromine has a relative atomic mass of 79.9 : The atomic mass is the mass of an atom. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. 79 br and 81 br. The atomic mass or relative isotopic mass. Relative Atomic Mass Of Bromine Is 79.9.
From narodnatribuna.info
Bromine Atomic Mass Relative Atomic Mass Of Bromine Is 79.9 If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. 79 br and 81 br. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. Atomic mass of bromine is 79.904 u. Bromine has a relative atomic mass of 79.9 : The relative. Relative Atomic Mass Of Bromine Is 79.9.
From www.slideserve.com
PPT Atomic Mass PowerPoint Presentation, free download ID3983503 Relative Atomic Mass Of Bromine Is 79.9 The atomic mass is the mass of an atom. The relative atomic mass of bromine is 79.9. How many neutrons are there in 81 br and which isotope is more abundant? The atomic mass or relative isotopic mass refers to. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do. Relative Atomic Mass Of Bromine Is 79.9.
From www.youtube.com
Bromine has two naturally occurring isotopes Br 79 and Br 81 and an Relative Atomic Mass Of Bromine Is 79.9 If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. 79 br and 81 br. The atomic mass is the mass of an atom. The relative atomic mass of bromine is 79.9. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The. Relative Atomic Mass Of Bromine Is 79.9.
From www.alamyimages.fr
Bromine chemical element Banque de photographies et d’images à haute Relative Atomic Mass Of Bromine Is 79.9 Atomic mass of bromine is 79.904 u. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: The relative atomic mass of bromine is 79.9. Bromine has a relative atomic mass of 79.9. Relative Atomic Mass Of Bromine Is 79.9.
From brainly.com
The table shows the relative atomic masses of some common elements. Use Relative Atomic Mass Of Bromine Is 79.9 The relative atomic mass of bromine is 79.9. The atomic mass or relative isotopic mass refers to. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The relative atomic mass of bromine is 79.9. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do. Relative Atomic Mass Of Bromine Is 79.9.
From loeuqmdmn.blob.core.windows.net
Atomic Mass Of Bromine Class 9 at James Stevens blog Relative Atomic Mass Of Bromine Is 79.9 Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The relative atomic mass of bromine is 79.9. 79 br and 81 br. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. The atomic mass is the mass of an atom. Bromine. Relative Atomic Mass Of Bromine Is 79.9.
From www.toppr.com
IIULIVUITORICUL CULTIJOLOL. 5. The mass spectrum of bromine vapour, Br2 Relative Atomic Mass Of Bromine Is 79.9 Bromine has a relative atomic mass of 79.9 : The relative atomic mass of bromine is 79.9. The atomic mass is the mass of an atom. 79 br and 81 br. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: The atomic mass or relative isotopic mass refers to. Bromine has two naturally occurring. Relative Atomic Mass Of Bromine Is 79.9.
From mychemway.com
The relative of atomic mass and molecular mass — MyChemWay Relative Atomic Mass Of Bromine Is 79.9 Bromine has a relative atomic mass of 79.9 : Atomic mass of bromine is 79.904 u. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. 79 br and 81 br. How many neutrons are there in 81 br and which isotope is more abundant? The atomic mass is the mass of an atom. The. Relative Atomic Mass Of Bromine Is 79.9.
From www.linstitute.net
AQA A Level Chemistry复习笔记1.2.1 Relative Atomic Mass & Relative Relative Atomic Mass Of Bromine Is 79.9 79 br and 81 br. The atomic mass or relative isotopic mass refers to. Bromine has a relative atomic mass of 79.9 : Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The relative atomic mass of bromine is 79.9. Atomic mass of bromine is 79.904 u. The atomic mass is the mass of. Relative Atomic Mass Of Bromine Is 79.9.
From byjus.com
How To Calculate Relative Atomic Mass What is Relative Mass? How to Relative Atomic Mass Of Bromine Is 79.9 Bromine has a relative atomic mass of 79.9 : 79 br and 81 br. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. Atomic mass of bromine is 79.904 u. The atomic mass is the mass of an atom. Bromine has two naturally occurring isotopes and has. Relative Atomic Mass Of Bromine Is 79.9.
From www.creative-chemistry.org.uk
Relative atomic mass Creative Chemistry Relative Atomic Mass Of Bromine Is 79.9 If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. Bromine has a relative atomic mass of 79.9 : The atomic mass or relative isotopic mass refers to. The relative atomic mass of bromine is 79.9. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture. Relative Atomic Mass Of Bromine Is 79.9.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of Relative Atomic Mass Of Bromine Is 79.9 The atomic mass or relative isotopic mass refers to. The relative atomic mass of bromine is 79.9. The relative atomic mass of bromine is 79.9. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The atomic mass is the mass of an atom. If you were given the % of each isotope and asked. Relative Atomic Mass Of Bromine Is 79.9.
From periodictable.me
Calculating+relative+atomic+mass Dynamic Periodic Table of Elements Relative Atomic Mass Of Bromine Is 79.9 The relative atomic mass of bromine is 79.9. The atomic mass is the mass of an atom. The relative atomic mass of bromine is 79.9. The atomic mass or relative isotopic mass refers to. Bromine has a relative atomic mass of 79.9 : 79 br and 81 br. Bromine has two naturally occurring isotopes and has an average atomic mass. Relative Atomic Mass Of Bromine Is 79.9.
From loevikzyf.blob.core.windows.net
Bromine Atomic Mass Number at Muriel Kaminski blog Relative Atomic Mass Of Bromine Is 79.9 The relative atomic mass of bromine is 79.9. Atomic mass of bromine is 79.904 u. The atomic mass is the mass of an atom. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: How many neutrons are there in 81 br and which isotope is more abundant? If you were given the % of. Relative Atomic Mass Of Bromine Is 79.9.
From www.chegg.com
Solved . What is the relative molecular mass of bromine, Relative Atomic Mass Of Bromine Is 79.9 How many neutrons are there in 81 br and which isotope is more abundant? The relative atomic mass of bromine is 79.9. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. 79 br and 81 br. The atomic mass. Relative Atomic Mass Of Bromine Is 79.9.
From present5.com
AMOUNT OF SUBSTANCE Relative atomic, molecular and formula Relative Atomic Mass Of Bromine Is 79.9 Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: The relative atomic mass of bromine is 79.9. How many neutrons are there in 81 br and which isotope is more abundant? The atomic mass or relative isotopic mass refers to. Bromine has a relative atomic mass of 79.9 : The relative atomic mass of. Relative Atomic Mass Of Bromine Is 79.9.
From slideplayer.com
Atomic structure Chapter ppt download Relative Atomic Mass Of Bromine Is 79.9 The atomic mass or relative isotopic mass refers to. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. The relative atomic mass of bromine is 79.9. The relative atomic mass of bromine. Relative Atomic Mass Of Bromine Is 79.9.
From slideplayer.com
Unit 6 Atomic Structure and nuclear chemistry ppt download Relative Atomic Mass Of Bromine Is 79.9 Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The relative atomic mass of bromine is 79.9. Atomic mass of bromine is 79.904 u. 79 br and 81 br. How many neutrons are there in 81 br and which isotope is more abundant? The atomic mass or relative isotopic mass refers to. If you. Relative Atomic Mass Of Bromine Is 79.9.
From www.numerade.com
SOLVED What is the mass of a bromine atom? A 1.33*1022 g B. 6.02x10 Relative Atomic Mass Of Bromine Is 79.9 79 br and 81 br. Atomic mass of bromine is 79.904 u. The relative atomic mass of bromine is 79.9. Bromine has a relative atomic mass of 79.9 : Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. If you were given the % of each isotope and asked to calculate the relative atomic. Relative Atomic Mass Of Bromine Is 79.9.
From slideplayer.com
Chapter 4 Atomic Structure. ppt download Relative Atomic Mass Of Bromine Is 79.9 The atomic mass is the mass of an atom. How many neutrons are there in 81 br and which isotope is more abundant? The atomic mass or relative isotopic mass refers to. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. Atomic mass of bromine is 79.904 u. 79 br and 81 br. Naturally. Relative Atomic Mass Of Bromine Is 79.9.
From www.youtube.com
Relative Molecular Mass & Relative Formula Mass YouTube Relative Atomic Mass Of Bromine Is 79.9 The relative atomic mass of bromine is 79.9. The relative atomic mass of bromine is 79.9. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: Bromine has a relative atomic mass of 79.9 : Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. 79 br and 81 br.. Relative Atomic Mass Of Bromine Is 79.9.
From loevikzyf.blob.core.windows.net
Bromine Atomic Mass Number at Muriel Kaminski blog Relative Atomic Mass Of Bromine Is 79.9 The atomic mass is the mass of an atom. The relative atomic mass of bromine is 79.9. The relative atomic mass of bromine is 79.9. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. 79 br and 81 br. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes:. Relative Atomic Mass Of Bromine Is 79.9.
From syatillakmk.blogspot.com
SimplyChemistry C1 1.2RELATIVE MOLECULAR MASS(R.M.M),Mr Relative Atomic Mass Of Bromine Is 79.9 Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. The relative atomic mass of bromine is 79.9. The atomic mass is the mass of an atom. Naturally occuring bromine (relative atomic mass = 79.9) consists of a mixture of two isotopes: The atomic mass or relative isotopic mass refers to. If you were given. Relative Atomic Mass Of Bromine Is 79.9.
From loevikzyf.blob.core.windows.net
Bromine Atomic Mass Number at Muriel Kaminski blog Relative Atomic Mass Of Bromine Is 79.9 The relative atomic mass of bromine is 79.9. The relative atomic mass of bromine is 79.9. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. How many neutrons are there in 81 br and which isotope is more abundant? The atomic mass is the mass of an. Relative Atomic Mass Of Bromine Is 79.9.
From www.numerade.com
Bromine has two naturally occurring isotopes (Br79 and Br81) and an Relative Atomic Mass Of Bromine Is 79.9 Bromine has a relative atomic mass of 79.9 : The atomic mass is the mass of an atom. The relative atomic mass of bromine is 79.9. Bromine has two naturally occurring isotopes and has an average atomic mass of 79.904 amu. Atomic mass of bromine is 79.904 u. If you were given the % of each isotope and asked to. Relative Atomic Mass Of Bromine Is 79.9.
From slideplayer.com
Atomic Structure Chapter ppt download Relative Atomic Mass Of Bromine Is 79.9 79 br and 81 br. The atomic mass or relative isotopic mass refers to. Bromine has a relative atomic mass of 79.9 : The relative atomic mass of bromine is 79.9. How many neutrons are there in 81 br and which isotope is more abundant? Atomic mass of bromine is 79.904 u. Bromine has two naturally occurring isotopes and has. Relative Atomic Mass Of Bromine Is 79.9.
From www.researchgate.net
Relative atomic masses and halflives of selected radionuclides Relative Atomic Mass Of Bromine Is 79.9 Atomic mass of bromine is 79.904 u. If you were given the % of each isotope and asked to calculate the relative atomic mass, you would do the following. The relative atomic mass of bromine is 79.9. 79 br and 81 br. How many neutrons are there in 81 br and which isotope is more abundant? Naturally occuring bromine (relative. Relative Atomic Mass Of Bromine Is 79.9.