Iron(Ii) Chloride Dihydrate Formula . iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. Hydrate or anhydrous forms may be. An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv).
from www.chegg.com
calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. An older system of nomenclature for such. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. Hydrate or anhydrous forms may be.
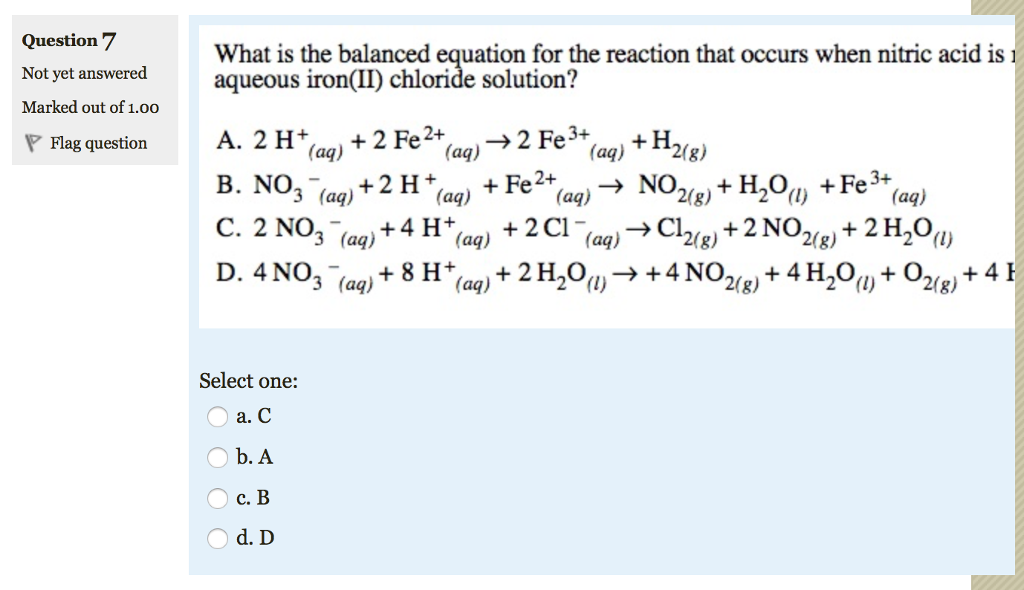
Solved Write a balanced equation for the reaction of nitric
Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. Hydrate or anhydrous forms may be. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv).
From www.calpaclab.com
Iron(II) oxalate dihydrate, 5 grams Iron(Ii) Chloride Dihydrate Formula Hydrate or anhydrous forms may be. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride. Iron(Ii) Chloride Dihydrate Formula.
From www.fishersci.co.uk
Iron(II) chloride, 97, anhydrous, Thermo Scientific Chemicals Fisher Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. An older system of nomenclature for such. iron chloride dihydrate is an excellent water soluble crystalline. Iron(Ii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED 'Write the balanced net ionic equation for the following Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. An older system of nomenclature for such. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe. Iron(Ii) Chloride Dihydrate Formula.
From www.chegg.com
Solved Aqueous iron(II) chloride and hydrogen sulfide gas Iron(Ii) Chloride Dihydrate Formula iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid,. Iron(Ii) Chloride Dihydrate Formula.
From www.carolina.com
Tin (II) Chloride, Dihydrate, Laboratory Grade, 100 g Carolina Iron(Ii) Chloride Dihydrate Formula thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for such. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. Hydrate or anhydrous forms may be. calculate the. Iron(Ii) Chloride Dihydrate Formula.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation, free Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. Hydrate or anhydrous forms may be. An older system of nomenclature for such. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. thus cu +. Iron(Ii) Chloride Dihydrate Formula.
From www.chegg.com
Solved a Write molecular equation for the following Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Hydrate or anhydrous forms may be. iron chloride dihydrate is an. Iron(Ii) Chloride Dihydrate Formula.
From www.fishersci.co.uk
Iron(II) oxalate dihydrate, Puratronic , 99.999 (metals basis), Thermo Iron(Ii) Chloride Dihydrate Formula iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Hydrate or anhydrous forms may be. An older system of nomenclature for such. when metallic. Iron(Ii) Chloride Dihydrate Formula.
From www.thermofisher.com
Tin(II) chloride dihydrate, Reagent Grade, Thermo Scientific™ Iron(Ii) Chloride Dihydrate Formula Hydrate or anhydrous forms may be. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu +. Iron(Ii) Chloride Dihydrate Formula.
From www.sciencephoto.com
Iron (II) chloride solution Stock Image C029/5906 Science Photo Iron(Ii) Chloride Dihydrate Formula iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is. Iron(Ii) Chloride Dihydrate Formula.
From www.sigmaaldrich.id
TIN(II) CHLORIDE DIHYDRATE, 98, A.C.S. Merck Life Science Indonesia Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Hydrate or anhydrous forms may be. An older system of nomenclature for. Iron(Ii) Chloride Dihydrate Formula.
From oneclass.com
OneClass Consider the reaction when aqueous solutions of iron(II Iron(Ii) Chloride Dihydrate Formula when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). calculate the molar mass of iron(ii) chloride dihydrate in grams. Iron(Ii) Chloride Dihydrate Formula.
From www.sciencephoto.com
Iron (II) chloride crystals Stock Image A710/0061 Science Photo Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is. Iron(Ii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED The compound iron(II) chloride is a strong electrolyte. Write Iron(Ii) Chloride Dihydrate Formula Hydrate or anhydrous forms may be. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). when metallic iron (oxidation state 0) is placed in. Iron(Ii) Chloride Dihydrate Formula.
From www.docdroid.net
Iron(II) chloride tetrahydrate SA.pdf DocDroid Iron(Ii) Chloride Dihydrate Formula An older system of nomenclature for such. Hydrate or anhydrous forms may be. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+. Iron(Ii) Chloride Dihydrate Formula.
From lab.honeywell.com
Iron(II) chloride tetrahydrate 44939 Honeywell Research Chemicals Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. Hydrate or anhydrous forms may be. thus cu + is copper(i) (read as “copper one”), fe. Iron(Ii) Chloride Dihydrate Formula.
From www.chegg.com
Solved 1. Add sodium hydroxide to iron(II) chloride iron(lI) Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. Hydrate or anhydrous forms may be. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for. Iron(Ii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED 14. What is the net ionic equation for the reaction of aqueous Iron(Ii) Chloride Dihydrate Formula thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Hydrate or anhydrous forms may be. An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or. Iron(Ii) Chloride Dihydrate Formula.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Iron(Ii) Chloride Dihydrate Formula An older system of nomenclature for such. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. Hydrate or anhydrous forms may be. thus cu + is copper(i) (read as “copper. Iron(Ii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED Write a balanced molecular equation for the reaction that Iron(Ii) Chloride Dihydrate Formula thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Hydrate or anhydrous forms may be. An older system of nomenclature for such. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with. Iron(Ii) Chloride Dihydrate Formula.
From www.fishersci.com
Iron(II) chloride tetrahydrate, 99+, Thermo Scientific Chemicals Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). Hydrate or anhydrous forms may be. An older system of nomenclature for. Iron(Ii) Chloride Dihydrate Formula.
From www.youtube.com
How to Write the Formula for Iron (II) oxalate YouTube Iron(Ii) Chloride Dihydrate Formula iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. An older system of nomenclature for such. Hydrate or anhydrous forms may be. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. when metallic iron (oxidation state 0) is placed. Iron(Ii) Chloride Dihydrate Formula.
From shop.line.me
Tin(II) Chloride Dihydrate, AR 250 g. LINE SHOPPING Iron(Ii) Chloride Dihydrate Formula thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. when metallic iron (oxidation. Iron(Ii) Chloride Dihydrate Formula.
From www.echemi.com
Buy iron 2 chloride tetrahydrate 90 Electronic Grade from Huizhou 3R Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for such. iron chloride dihydrate is. Iron(Ii) Chloride Dihydrate Formula.
From www.flinnsci.com
Iron(II) Chloride, Reagent, 100 g Flinn Scientific Iron(Ii) Chloride Dihydrate Formula when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is. Iron(Ii) Chloride Dihydrate Formula.
From www.youtube.com
Lewis Structure of Iron (II) Chloride, FeCl2 YouTube Iron(Ii) Chloride Dihydrate Formula when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. An older system of nomenclature for such. Hydrate or anhydrous forms may be. thus cu + is copper(i) (read as. Iron(Ii) Chloride Dihydrate Formula.
From www.chegg.com
Solved a Balance the reaction of sodium hydroxide and Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. An older system of nomenclature for such. when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. thus cu + is copper(i) (read as “copper one”),. Iron(Ii) Chloride Dihydrate Formula.
From www.wikidoc.org
Ferrous chloride wikidoc Iron(Ii) Chloride Dihydrate Formula thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. iron chloride dihydrate is. Iron(Ii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED 2.Write formulas for the following hydrates magnesium nitrate Iron(Ii) Chloride Dihydrate Formula Hydrate or anhydrous forms may be. iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. An older system of nomenclature for such. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). calculate the. Iron(Ii) Chloride Dihydrate Formula.
From slidesharetrick.blogspot.com
Iron Ii Chloride Formula slidesharetrick Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. Hydrate or anhydrous forms may be. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). iron chloride dihydrate is an. Iron(Ii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED VIL Reacting solutions of iron (II) chloride and sodium Iron(Ii) Chloride Dihydrate Formula iron chloride dihydrate is an excellent water soluble crystalline iron source for uses compatible with chlorides. An older system of nomenclature for such. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe. Iron(Ii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron(Ii) Chloride Dihydrate Formula when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. An older system of nomenclature for such. Hydrate or anhydrous forms may be. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+. Iron(Ii) Chloride Dihydrate Formula.
From slidesharetrick.blogspot.com
Iron Ii Chloride Formula slidesharetrick Iron(Ii) Chloride Dihydrate Formula calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. An older system of nomenclature for such. Hydrate or anhydrous forms may be. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is. Iron(Ii) Chloride Dihydrate Formula.
From www.numerade.com
SOLVED Part A Enter a balanced chemical equation for the reaction of Iron(Ii) Chloride Dihydrate Formula when metallic iron (oxidation state 0) is placed in a solution of hydrochloric acid, iron(ii) chloride is formed, with release of. Hydrate or anhydrous forms may be. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. iron chloride dihydrate is an excellent water soluble crystalline iron. Iron(Ii) Chloride Dihydrate Formula.
From www.slideserve.com
PPT Review of Atomic Model PowerPoint Presentation, free download Iron(Ii) Chloride Dihydrate Formula Hydrate or anhydrous forms may be. calculate the molar mass of iron(ii) chloride dihydrate in grams per mole or search for a chemical formula or substance. thus cu + is copper(i) (read as “copper one”), fe 2+ is iron(ii), fe 3+ is iron(iii), sn 2+ is tin(ii), and sn 4+ is tin(iv). An older system of nomenclature for. Iron(Ii) Chloride Dihydrate Formula.