Nitrogen Atmosphere Chemistry . nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: Nitrogen, the most abundant component, has accumulated over. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. N 2 forms about 78% of earth's. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. the atmospheric gases fall into three abundance categories: Water vapour condensed to form the. the early atmosphere was mainly carbon dioxide and water vapour.
from www.vedantu.com
at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. the early atmosphere was mainly carbon dioxide and water vapour. the atmospheric gases fall into three abundance categories: nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: Nitrogen, the most abundant component, has accumulated over. Water vapour condensed to form the. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. N 2 forms about 78% of earth's.
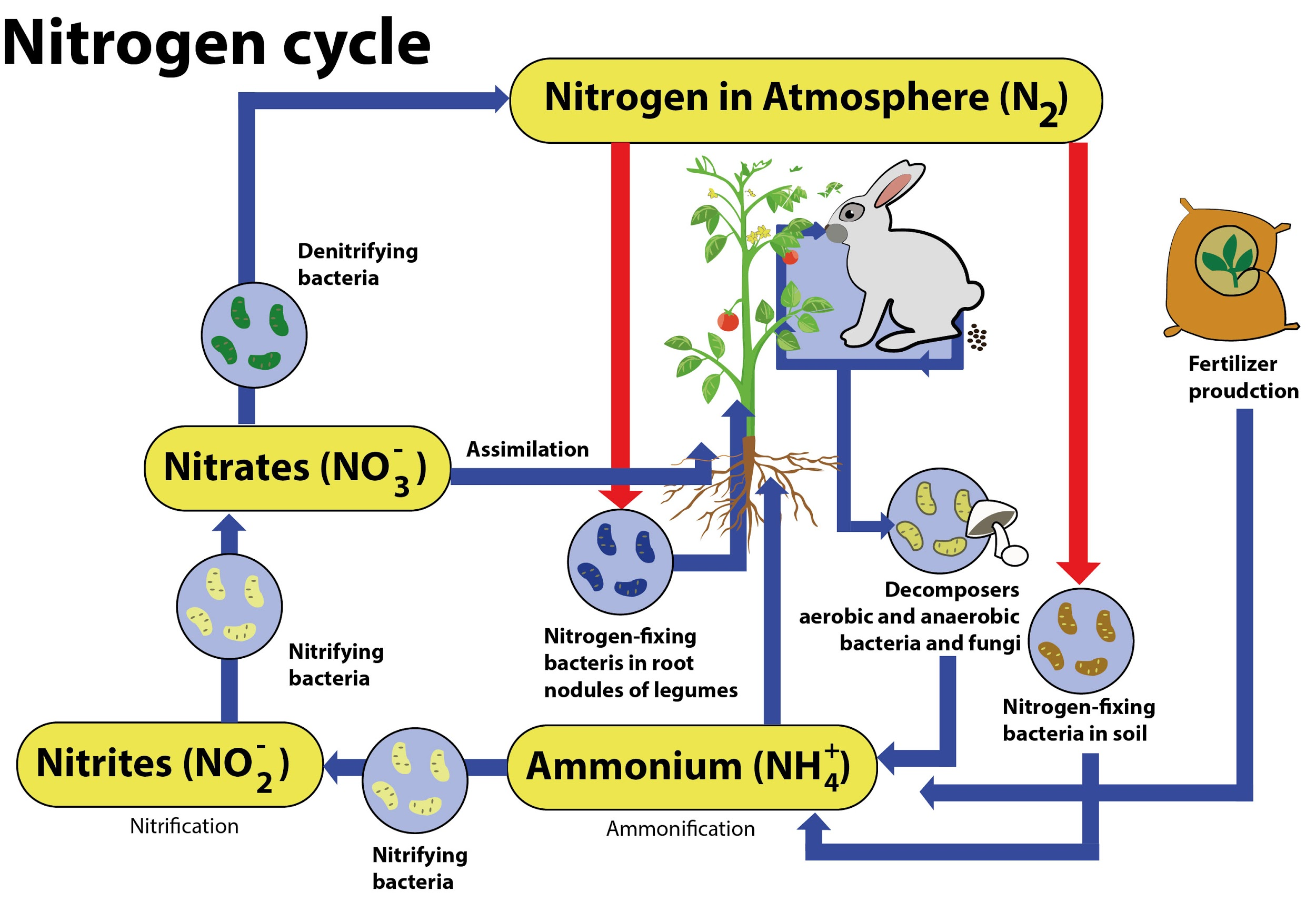
Describe the nitrogen cycle with the help of a diagram.
Nitrogen Atmosphere Chemistry the atmospheric gases fall into three abundance categories: nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: the atmospheric gases fall into three abundance categories: N 2 forms about 78% of earth's. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. the early atmosphere was mainly carbon dioxide and water vapour. Water vapour condensed to form the. Nitrogen, the most abundant component, has accumulated over. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen.
From www.chemeurope.com
NitrogenContaining Atmospheric Pollutants Nitrogen Atmosphere Chemistry Water vapour condensed to form the. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. N 2 forms about 78% of earth's. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at. Nitrogen Atmosphere Chemistry.
From study.com
Nitrogen Fixation Process Overview & Types Lesson Nitrogen Atmosphere Chemistry at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: the atmospheric gases fall into three abundance categories: about 90% of the nitrogen produced today is used to provide an inert atmosphere. Nitrogen Atmosphere Chemistry.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID Nitrogen Atmosphere Chemistry about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. N 2 forms about 78% of earth's. the early atmosphere was mainly carbon dioxide and water vapour. Nitrogen, the most abundant component, has accumulated over. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the. Nitrogen Atmosphere Chemistry.
From www.earth.com
Why is the Nitrogen Cycle So Important? Nitrogen Atmosphere Chemistry about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. the atmospheric gases fall into three abundance categories: Nitrogen, the most abundant component, has accumulated over. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic. Nitrogen Atmosphere Chemistry.
From www.researchgate.net
DSC thermograms of CEMs 3a and 3b in a nitrogen atmosphere. The Nitrogen Atmosphere Chemistry Water vapour condensed to form the. N 2 forms about 78% of earth's. Nitrogen, the most abundant component, has accumulated over. the early atmosphere was mainly carbon dioxide and water vapour. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. nitrogen dilutes oxygen and prevents. Nitrogen Atmosphere Chemistry.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID Nitrogen Atmosphere Chemistry the atmospheric gases fall into three abundance categories: Water vapour condensed to form the. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: about 90% of the nitrogen produced today is. Nitrogen Atmosphere Chemistry.
From www.science-sparks.com
What is the Nitrogen Cycle? Science for Kids Nitrogen Atmosphere Chemistry at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. Water vapour condensed to form the. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. nitrogen compounds are produced from atmospheric dinitrogen (n 2). Nitrogen Atmosphere Chemistry.
From stock.adobe.com
The nitrogen cycle showing relationship between the three main nitrogen Nitrogen Atmosphere Chemistry nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: N 2 forms about 78% of earth's. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. the atmospheric gases fall into three abundance categories: at standard temperature and pressure, two atoms. Nitrogen Atmosphere Chemistry.
From www.vectorstock.com
The composition of the atmosphere Nitrogen carbon Vector Image Nitrogen Atmosphere Chemistry about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. the atmospheric gases fall into three abundance categories: nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: N 2 forms about 78% of earth's. at standard temperature and pressure, two atoms. Nitrogen Atmosphere Chemistry.
From www.dreamstime.com
N2 Nitrogen Gas is the Main Constituent of the Earth S Atmosphere Nitrogen Atmosphere Chemistry about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. Water vapour condensed to form the. Nitrogen, the most abundant component, has accumulated over. the atmospheric gases fall into three abundance categories: N 2 forms about 78% of earth's. nitrogen compounds are produced from atmospheric dinitrogen. Nitrogen Atmosphere Chemistry.
From www.slideserve.com
PPT Nitrogen Chemistry in Titan’s Upper Atmosphere PowerPoint Nitrogen Atmosphere Chemistry nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. Water vapour. Nitrogen Atmosphere Chemistry.
From courses.lumenlearning.com
Occurrence, Preparation, and Properties of Nitrogen Chemistry for Majors Nitrogen Atmosphere Chemistry nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. the early atmosphere was mainly carbon dioxide and water vapour. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: the atmospheric gases fall into three abundance categories: Water vapour condensed to. Nitrogen Atmosphere Chemistry.
From www.earth.com
Why is the Nitrogen Cycle So Important? Nitrogen Atmosphere Chemistry at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen. Nitrogen Atmosphere Chemistry.
From www.chemix-chemistry-software.com
Nitrogen phase diagram Nitrogen Atmosphere Chemistry nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: the early atmosphere was mainly carbon dioxide and water vapour. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. the atmospheric gases fall into three abundance categories: Nitrogen, the most abundant. Nitrogen Atmosphere Chemistry.
From www.slideserve.com
PPT Nitrogen Cycle PowerPoint Presentation, free download ID1992023 Nitrogen Atmosphere Chemistry about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. the early atmosphere was mainly carbon dioxide and water vapour. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. nitrogen compounds are produced. Nitrogen Atmosphere Chemistry.
From www.britannica.com
nitrogen Definition, Symbol, Uses, Properties, Atomic Number, & Facts Nitrogen Atmosphere Chemistry Nitrogen, the most abundant component, has accumulated over. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. the atmospheric gases fall into three abundance. Nitrogen Atmosphere Chemistry.
From www.vedantu.com
Describe the nitrogen cycle with the help of a diagram. Nitrogen Atmosphere Chemistry nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: N 2 forms about 78% of earth's. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. the early atmosphere was mainly carbon dioxide and water vapour. the atmospheric gases fall into. Nitrogen Atmosphere Chemistry.
From www.careerpower.in
Nitrogen Cycle Definition, Process and Importance Nitrogen Atmosphere Chemistry Nitrogen, the most abundant component, has accumulated over. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. N 2 forms about 78% of earth's. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. Water. Nitrogen Atmosphere Chemistry.
From www.vectorstock.com
Diagram representation of the element nitrogen Vector Image Nitrogen Atmosphere Chemistry Nitrogen, the most abundant component, has accumulated over. the atmospheric gases fall into three abundance categories: at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are. Nitrogen Atmosphere Chemistry.
From socratic.org
How does nitrogen cycle through the biosphere? Socratic Nitrogen Atmosphere Chemistry nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. Nitrogen, the most abundant component, has accumulated over. the early atmosphere was mainly carbon dioxide. Nitrogen Atmosphere Chemistry.
From sandersdoreas.blogspot.com
Nitrogen Cycle Easy Diagram Nitrogen Cycle Diagram Sanders Doreas Nitrogen Atmosphere Chemistry Nitrogen, the most abundant component, has accumulated over. Water vapour condensed to form the. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. at standard temperature and pressure, two atoms of the. Nitrogen Atmosphere Chemistry.
From reefnation.com
The Nitrogen Cycle Revisited Nitrogen Atmosphere Chemistry the atmospheric gases fall into three abundance categories: Nitrogen, the most abundant component, has accumulated over. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. N 2 forms about 78% of earth's. Water vapour condensed to form the. nitrogen dilutes oxygen and prevents rapid or. Nitrogen Atmosphere Chemistry.
From notesforbiology.com
Nitrogen Cycle Steps And Significance Biology Notes Nitrogen Atmosphere Chemistry about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. the atmospheric gases fall into three abundance categories: nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: the early atmosphere was mainly carbon dioxide and water vapour. Water vapour condensed to. Nitrogen Atmosphere Chemistry.
From www.teachoo.com
Nitrogen Cycle Diagram with Steps Explained Teachoo Concepts Nitrogen Atmosphere Chemistry nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. the early atmosphere was mainly carbon dioxide and water vapour. the atmospheric gases fall into three abundance categories: Nitrogen, the most abundant component, has accumulated over. N 2 forms about 78% of earth's. nitrogen compounds. Nitrogen Atmosphere Chemistry.
From www.britannica.com
nitrogen fixation Definition, Process, Examples, Types, & Facts Nitrogen Atmosphere Chemistry N 2 forms about 78% of earth's. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. Nitrogen, the most abundant component, has accumulated over. . Nitrogen Atmosphere Chemistry.
From sciencetallis.weebly.com
9. Chemistry of the Atmosphere THOMAS TALLIS SCIENCE Nitrogen Atmosphere Chemistry N 2 forms about 78% of earth's. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. the early atmosphere was mainly carbon dioxide and water vapour. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that. Nitrogen Atmosphere Chemistry.
From revisechemistry.uk
Chemistry of the Atmosphere AQA C9 revisechemistry.uk Nitrogen Atmosphere Chemistry the atmospheric gases fall into three abundance categories: N 2 forms about 78% of earth's. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. Nitrogen, the most abundant component, has accumulated over. about 90% of the nitrogen produced today is used to provide an inert. Nitrogen Atmosphere Chemistry.
From studylib.net
Chapter 17 The Atmosphere • Composition of the • 78 Nitrogen Nitrogen Atmosphere Chemistry at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. the atmospheric gases fall into three abundance categories: nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: the early atmosphere was mainly carbon dioxide and water vapour. N 2 forms about. Nitrogen Atmosphere Chemistry.
From unacademy.com
What is nitrogen cycle By Unacademy Nitrogen Atmosphere Chemistry the atmospheric gases fall into three abundance categories: N 2 forms about 78% of earth's. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: Nitrogen, the most abundant component, has accumulated over. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen.. Nitrogen Atmosphere Chemistry.
From preschem.blogspot.com
Presentation College Chemistry Mr Adrian Ramlal's Class! The Nitrogen Nitrogen Atmosphere Chemistry nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: the early atmosphere was mainly carbon dioxide and water vapour. N 2 forms about 78% of earth's. Nitrogen, the most abundant component, has accumulated over. about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that. Nitrogen Atmosphere Chemistry.
From www.dreamstime.com
N2 Nitrogen Gas is the Main Constituent of the Earth`s Atmosphere Nitrogen Atmosphere Chemistry nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. N 2 forms about 78% of earth's. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. nitrogen compounds are produced from atmospheric dinitrogen (n. Nitrogen Atmosphere Chemistry.
From mavink.com
Draw And Label The Nitrogen Cycle Nitrogen Atmosphere Chemistry nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. Nitrogen, the most abundant component, has accumulated over. nitrogen compounds are produced from atmospheric dinitrogen. Nitrogen Atmosphere Chemistry.
From personal.ems.psu.edu
Chapter 4 Nitrogen Atmosphere Chemistry at standard temperature and pressure, two atoms of the element bond to form n 2, a colourless and odourless diatomic gas. Water vapour condensed to form the. nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant of. about 90% of the nitrogen produced today is used. Nitrogen Atmosphere Chemistry.
From mavink.com
Diagram Of Nitrogen Cycle Nitrogen Atmosphere Chemistry Water vapour condensed to form the. Nitrogen, the most abundant component, has accumulated over. the early atmosphere was mainly carbon dioxide and water vapour. nitrogen compounds are produced from atmospheric dinitrogen (n 2) by the following processes: nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the earth's surface, as oxygen gas is a necessary reactant. Nitrogen Atmosphere Chemistry.
From www.britannica.com
How the Nitrogen Cycle Works Britannica Nitrogen Atmosphere Chemistry about 90% of the nitrogen produced today is used to provide an inert atmosphere for processes or reactions that are oxygen. Water vapour condensed to form the. the atmospheric gases fall into three abundance categories: the early atmosphere was mainly carbon dioxide and water vapour. at standard temperature and pressure, two atoms of the element bond. Nitrogen Atmosphere Chemistry.