Lab Notebook Fda . It provides questions and answers on topics such. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Compare different data creation, storage and retrieval options, and download a free scorecard template. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks.
from labnotebook.app
This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. It provides questions and answers on topics such. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. Compare different data creation, storage and retrieval options, and download a free scorecard template. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks.
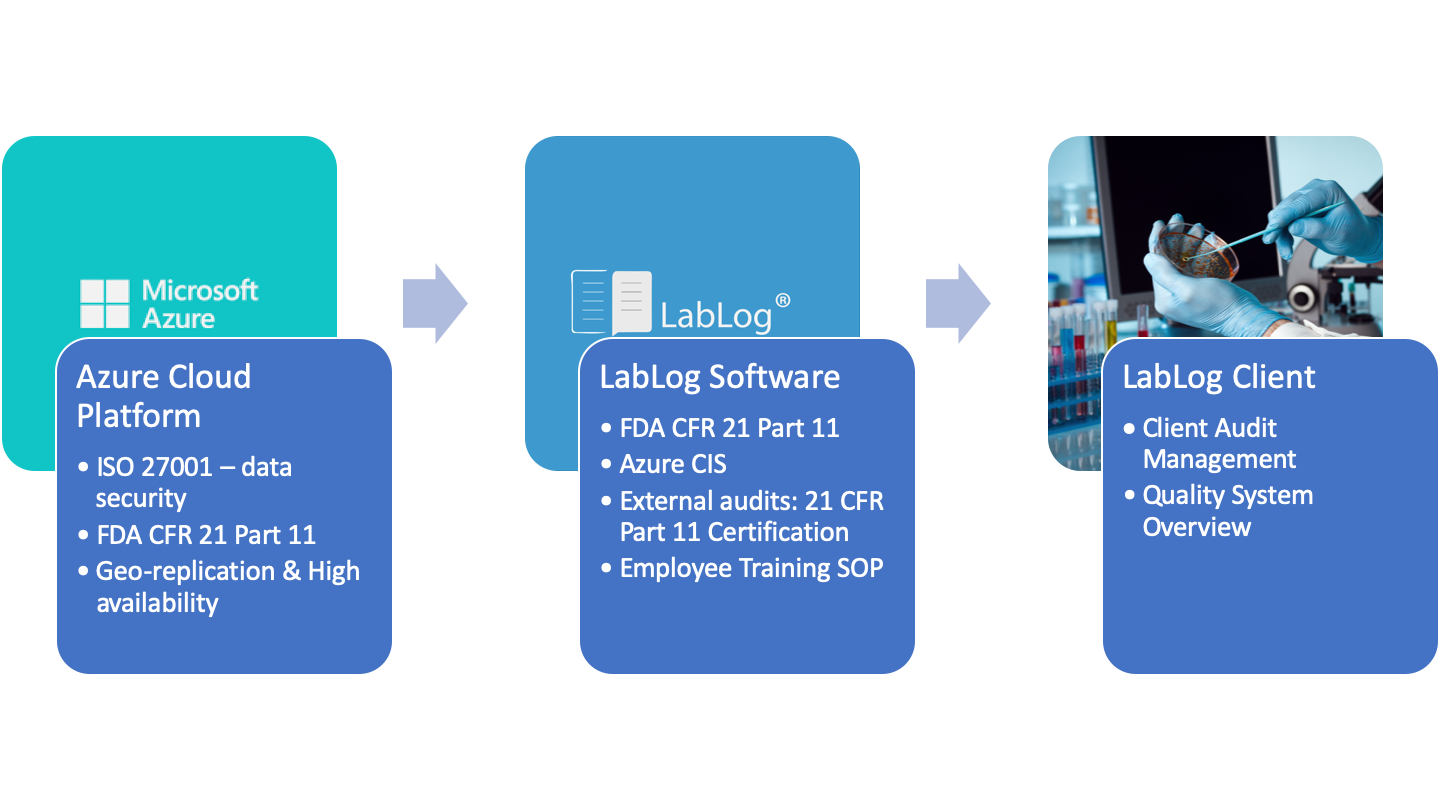
The AllInOne Solution for FDA Electronic Records Compliance LabLog
Lab Notebook Fda This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. It provides questions and answers on topics such. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Compare different data creation, storage and retrieval options, and download a free scorecard template. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,.
From info.recordsforce.com
Biotechnology Lab Notebook Case Study Lab Notebook Fda Compare different data creation, storage and retrieval options, and download a free scorecard template. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,.. Lab Notebook Fda.
From www.slideserve.com
PPT Lab Notebooks + Procedure PowerPoint Presentation, free download Lab Notebook Fda This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. It provides questions and answers on topics such. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. Electronic laboratory notebooks (elns) are computer systems. Lab Notebook Fda.
From snco.com
3001HCHZ Green Laboratory Notebook Scientific Notebook Company Lab Notebook Fda It provides questions and answers on topics such. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. This guidance clarifies the role. Lab Notebook Fda.
From studylib.net
Laboratory Notebook Guidelines Lab Notebook Fda This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. It provides questions and answers on topics such. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. Compare different data creation, storage and retrieval. Lab Notebook Fda.
From snco.com
3001HCHZ laboratory notebook Scientific Notebook Company Lab Notebook Fda Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that. Lab Notebook Fda.
From www.youtube.com
What is a Lab Notebook?! YouTube Lab Notebook Fda This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This guidance clarifies the role of data integrity in cgmp for drugs, as required by. Lab Notebook Fda.
From www.youtube.com
Lab Notebook Guidelines YouTube Lab Notebook Fda This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. It provides questions and answers on topics such. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. Learn how to choose the best. Lab Notebook Fda.
From www.dreamstime.com
Notebook with FDA APPROVED stock image. Image of safe 233734333 Lab Notebook Fda Compare different data creation, storage and retrieval options, and download a free scorecard template. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. This draft guidance provides industry with. Lab Notebook Fda.
From snco.com
3001CP Laboratory Notebook Scientific Notebook Company Lab Notebook Fda This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Compare different data creation, storage and retrieval options, and download a free scorecard template. It provides questions and answers on topics such. Learn how to choose the best eln for your research from this comprehensive guide by labfolder,. Lab Notebook Fda.
From www.labfolder.com
How to Keep a Lab Notebook labfolder Lab Notebook Fda Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Learn how to choose the best eln for your research from this comprehensive guide. Lab Notebook Fda.
From www.pdffiller.com
Laboratory notebook sample incorporating a chemical Doc Template Lab Notebook Fda Compare different data creation, storage and retrieval options, and download a free scorecard template. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Electronic laboratory notebooks (elns) are computer. Lab Notebook Fda.
From plantae.org
The Lab Notebook A Researcher’s Best Friend Plantae Lab Notebook Fda Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Learn how to choose the best eln for your research from this comprehensive guide. Lab Notebook Fda.
From studylib.net
Lab Notebook Guidelines Lab Notebook Fda It provides questions and answers on topics such. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. Compare different data creation, storage and retrieval options, and download a free scorecard template. This draft guidance provides industry with fda's current thinking on data integrity and compliance with. Lab Notebook Fda.
From snco.com
3001HZ Laboratory Notebook Blue covers Scientific Notebook Company Lab Notebook Fda It provides questions and answers on topics such. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. Compare different data creation, storage and retrieval options, and download a free scorecard template. This guidance clarifies the role of data integrity in cgmp for drugs, as required. Lab Notebook Fda.
From www.goldbio.com
15 Laboratory Notebook Tips to Help with your Research Manuscript GoldBio Lab Notebook Fda This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Compare different data creation, storage and retrieval options, and download a free scorecard template. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. It provides questions and answers on topics. Lab Notebook Fda.
From www.capterra.co.uk
SciNote Electronic Lab Notebook Pricing, Cost & Reviews Capterra UK 2021 Lab Notebook Fda This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. It provides questions and answers on topics such. Compare different data creation, storage and retrieval options, and download a free. Lab Notebook Fda.
From www.walmart.com
Laboratory Notebook, Research Lab Notebook Fda Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Compare different data creation, storage and retrieval options, and download a free scorecard template. It. Lab Notebook Fda.
From masterbundles.com
Laboratory Notebook Journal KDP Interior MasterBundles Lab Notebook Fda Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that. Lab Notebook Fda.
From www.kubookstore.com
Lab Notebook, 75Sheet Set (Spiral) Lab Notebook Fda Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. It provides questions and answers on topics such. This guidance clarifies the role. Lab Notebook Fda.
From snco.com
Student Lab Notebooks Scientific Notebook Company Lab Notebook Fda This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet. Lab Notebook Fda.
From midsci.com
Lab Notebooks Lab Notebook Fda Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. It provides questions and answers on topics such. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. This draft guidance provides industry. Lab Notebook Fda.
From www.scribd.com
Lab Notebook Guide (1) Experiment Notebook Lab Notebook Fda This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Compare different data creation, storage and retrieval options, and download a free scorecard template. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. It provides questions and answers. Lab Notebook Fda.
From www.integle-eln.com
InELN, A professional Electronic Lab Notebook for private deployment Lab Notebook Fda It provides questions and answers on topics such. Compare different data creation, storage and retrieval options, and download a free scorecard template. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. This guidance clarifies the role of data integrity in cgmp for drugs, as required. Lab Notebook Fda.
From www.uncountable.com
Free Guide Electronic Laboratory Notebooks ELN Guide Lab Notebook Fda It provides questions and answers on topics such. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. Compare different data creation, storage and retrieval options, and download a free scorecard template. This draft guidance provides industry with fda's current thinking on data integrity and compliance. Lab Notebook Fda.
From snco.com
3001HC96P Hard Cover Laboratory Notebook Scientific Notebook Company Lab Notebook Fda It provides questions and answers on topics such. Compare different data creation, storage and retrieval options, and download a free scorecard template. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This draft guidance provides industry with fda's current thinking on data integrity and compliance with. Lab Notebook Fda.
From www.rpicorp.com
156321 Nalgene Laboratory Notebook with Ruled Lines, Conventional Lab Notebook Fda It provides questions and answers on topics such. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. This draft guidance provides industry with fda's current thinking on data integrity. Lab Notebook Fda.
From snco.com
3001HC Black Cover Laboratory Notebook Scientific Notebook Company Lab Notebook Fda This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. It provides questions and answers on topics such. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving,. Lab Notebook Fda.
From snco.com
3001HC Black Cover Laboratory Notebook Scientific Notebook Company Lab Notebook Fda Compare different data creation, storage and retrieval options, and download a free scorecard template. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. It provides questions and answers on topics such. This guidance clarifies the role of data integrity in cgmp for drugs, as required. Lab Notebook Fda.
From www.researchgate.net
(PDF) Analysis and Implementation of an Electronic Laboratory Notebook Lab Notebook Fda Compare different data creation, storage and retrieval options, and download a free scorecard template. This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,.. Lab Notebook Fda.
From studylib.net
Lab Notebook Guidelines 20202021 (1) Lab Notebook Fda This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. Learn how to choose the best eln for your research from this comprehensive guide. Lab Notebook Fda.
From snco.com
Laboratory Notebooks HighQuality Bound Lab Notebooks Lab Notebook Fda Compare different data creation, storage and retrieval options, and download a free scorecard template. It provides questions and answers on topics such. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. Learn how to choose the best eln for your research from this comprehensive guide. Lab Notebook Fda.
From www.dreamstime.com
Notebook Written with FDA & X28;Food and Drug Administration. Stock Lab Notebook Fda Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. It provides questions and answers on topics such. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Learn how to choose the best eln for your research from. Lab Notebook Fda.
From snco.com
2001HC Green Cover laboratory notebook Scientific Notebook Company Lab Notebook Fda Compare different data creation, storage and retrieval options, and download a free scorecard template. Electronic laboratory notebooks (elns) are computer systems used for creating, storing, retrieving, and sharing fully electronic records in ways that meet all legal, regulatory,. It provides questions and answers on topics such. Learn how to choose the best eln for your research from this comprehensive guide. Lab Notebook Fda.
From snco.com
3001HCHZ Green Laboratory Notebook Scientific Notebook Company Lab Notebook Fda Learn how to choose the best eln for your research from this comprehensive guide by labfolder, a company that develops electronic lab notebooks. Compare different data creation, storage and retrieval options, and download a free scorecard template. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. Electronic laboratory notebooks (elns) are computer. Lab Notebook Fda.
From labnotebook.app
The AllInOne Solution for FDA Electronic Records Compliance LabLog Lab Notebook Fda This draft guidance provides industry with fda's current thinking on data integrity and compliance with current good manufacturing practice (cgmp) for pharmaceuticals. This guidance clarifies the role of data integrity in cgmp for drugs, as required by fda regulations. It provides questions and answers on topics such. Compare different data creation, storage and retrieval options, and download a free scorecard. Lab Notebook Fda.