Medical Device Approval Lebanon . Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The ministry of public health regulates medical devices in lebanon. The eu mdr 2017/745 came into effect in may 2021. The two bodies regulating medical device registration in lebanon are the drug import and export department and. The medical device market in lebanon mostly relies on. All medical devices are regulated by the ministry of public health (moph). Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow.
from slidetodoc.com
All medical devices are regulated by the ministry of public health (moph). The ministry of public health regulates medical devices in lebanon. The medical device market in lebanon mostly relies on. Access the list of regulatied products, classifications and approval. The two bodies regulating medical device registration in lebanon are the drug import and export department and. All medical devices certified under the previous medical device directive (mdd) must. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The eu mdr 2017/745 came into effect in may 2021.
The FDA Approval Process for New Devices Roxana
Medical Device Approval Lebanon The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. All medical devices certified under the previous medical device directive (mdd) must. Access the list of regulatied products, classifications and approval. The two bodies regulating medical device registration in lebanon are the drug import and export department and. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The medical device market in lebanon mostly relies on. All medical devices are regulated by the ministry of public health (moph). The eu mdr 2017/745 came into effect in may 2021. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The ministry of public health regulates medical devices in lebanon.
From credevo.com
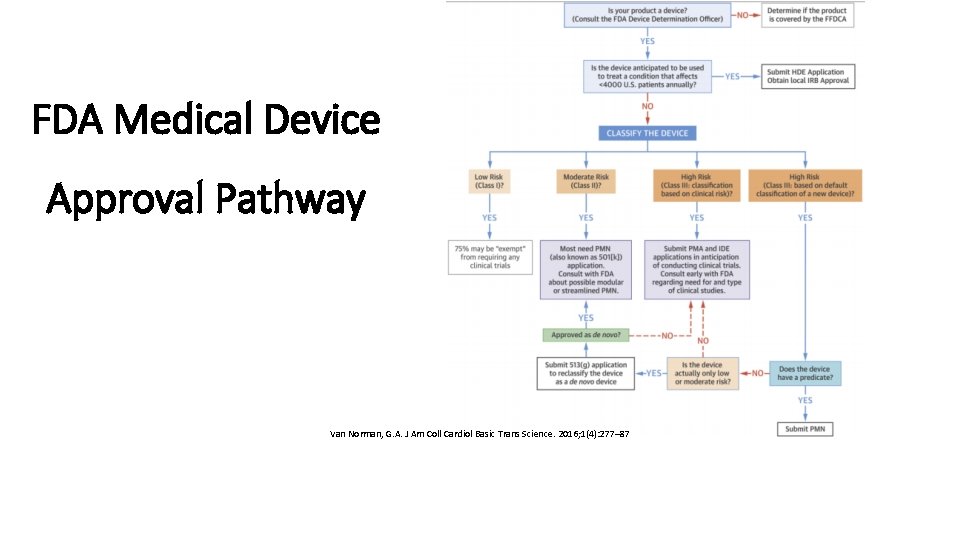
Medical Device Market Approval Process in the United States Credevo Medical Device Approval Lebanon Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. Lebanon is in a great location in the middle east, making it a good place for medical device companies to. Medical Device Approval Lebanon.
From www.researchgate.net
Medical devices approval pathway in US [17]. Download Scientific Diagram Medical Device Approval Lebanon The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. All medical devices are regulated by the ministry of public health (moph). Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The ministry of public health regulates medical devices. Medical Device Approval Lebanon.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Medical Device Approval Lebanon The ministry of public health regulates medical devices in lebanon. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The two bodies regulating medical device registration in lebanon are the drug import and export department and. All medical devices certified under the previous medical device directive (mdd) must. Access the. Medical Device Approval Lebanon.
From www.youtube.com
Process of Registration Medical Devices in India Guideline for Medical Device Approval Lebanon The medical device market in lebanon mostly relies on. Access the list of regulatied products, classifications and approval. The two bodies regulating medical device registration in lebanon are the drug import and export department and. The eu mdr 2017/745 came into effect in may 2021. Lebanon is in a great location in the middle east, making it a good place. Medical Device Approval Lebanon.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Device Approval Lebanon The medical device market in lebanon mostly relies on. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The ministry of public health regulates medical devices in lebanon. The eu mdr 2017/745 came into effect in may 2021. Access the list of regulatied products, classifications and approval. All medical devices. Medical Device Approval Lebanon.
From credevo.com
Medical Device Regulatory And Approval In Malaysia Credevo Articles Medical Device Approval Lebanon Access the list of regulatied products, classifications and approval. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The eu mdr 2017/745 came into effect in may 2021. All medical devices are regulated by the ministry of public health (moph). The ministry of health (moph), in cooperation with. Medical Device Approval Lebanon.
From pdfslide.net
(PPT) + Medical Devices Approval Process. + Objectives Define a medical Medical Device Approval Lebanon Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits. Medical Device Approval Lebanon.
From www.vrogue.co
Eu Ivd Approval Process For Medical Devices vrogue.co Medical Device Approval Lebanon The medical device market in lebanon mostly relies on. Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The ministry of health (moph), in cooperation with the. Medical Device Approval Lebanon.
From credevo.com
Medical Device Market Approval Process in the United States Credevo Medical Device Approval Lebanon The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The ministry of public health regulates medical devices in lebanon. All medical devices certified under the previous medical device directive. Medical Device Approval Lebanon.
From nextern.com
A Look at the Medical Device Approval Process Nextern Medical Device Approval Lebanon All medical devices are regulated by the ministry of public health (moph). Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. The two bodies regulating medical device registration in lebanon are the drug import and export department and. The eu mdr 2017/745 came into effect in may 2021.. Medical Device Approval Lebanon.
From www.vrogue.co
Five Years Roadmap Of Medical Device Approval From Fd vrogue.co Medical Device Approval Lebanon Access the list of regulatied products, classifications and approval. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The ministry of public health regulates medical devices in lebanon. The. Medical Device Approval Lebanon.
From operonstrategist.com
CE Approval Process for Medical Devices Medical Device Approval Lebanon Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The two bodies regulating medical device registration in lebanon are the drug import and export department and. The eu mdr 2017/745 came into effect in may 2021. All medical devices are regulated by the ministry of public health (moph).. Medical Device Approval Lebanon.
From twitter.com
Dr Kalpesh hegde on Twitter "FDA Medical devices approval process in 5 Medical Device Approval Lebanon The medical device market in lebanon mostly relies on. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. All medical devices are regulated by the ministry of public health (moph). The two bodies regulating medical device registration in lebanon are the drug import and export department and. Access. Medical Device Approval Lebanon.
From operonstrategist.com
Medical Device Regulatory Consulting CDSCO Approval Process for Medical Device Approval Lebanon The two bodies regulating medical device registration in lebanon are the drug import and export department and. Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. All medical devices. Medical Device Approval Lebanon.
From slidetodoc.com
The FDA Approval Process for New Devices Roxana Medical Device Approval Lebanon The two bodies regulating medical device registration in lebanon are the drug import and export department and. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The eu mdr 2017/745 came into effect in may 2021. All medical devices certified under the previous medical device directive (mdd) must.. Medical Device Approval Lebanon.
From www.presentationeze.com
Medical Device Approval European Regulatory ProcessPresentationEZE Medical Device Approval Lebanon The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. Access the list of regulatied products, classifications and approval. The ministry of public health regulates medical devices in lebanon. The eu mdr 2017/745 came into effect in may 2021. The medical device market in lebanon mostly relies on. The two bodies. Medical Device Approval Lebanon.
From credevo.com
Japan Medical Device Approval Process Credevo Articles Medical Device Approval Lebanon The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The eu mdr 2017/745 came into effect in may 2021. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. All medical devices are regulated by the ministry of public. Medical Device Approval Lebanon.
From www.jtp.co.jp
Medical Devices Approval Process in Japan JTP Co., Ltd. Medical Device Approval Lebanon The medical device market in lebanon mostly relies on. All medical devices certified under the previous medical device directive (mdd) must. The eu mdr 2017/745 came into effect in may 2021. Access the list of regulatied products, classifications and approval. The ministry of public health regulates medical devices in lebanon. Lebanon is in a great location in the middle east,. Medical Device Approval Lebanon.
From credevo.com
Japan Medical Device Approval Process Credevo Articles Medical Device Approval Lebanon The medical device market in lebanon mostly relies on. Access the list of regulatied products, classifications and approval. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The two bodies regulating medical device registration in lebanon are the drug import and export department and. The ministry of health. Medical Device Approval Lebanon.
From www.nemko.com
Steps for achieving medical device approval Medical Device Approval Lebanon Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. All medical devices are regulated by the ministry of public health (moph). The ministry of public health regulates medical devices in lebanon. The eu mdr 2017/745 came into effect in may 2021. The medical device market in lebanon mostly. Medical Device Approval Lebanon.
From www.youtube.com
Medical Device Regulations / FDA Approval YouTube Medical Device Approval Lebanon All medical devices are regulated by the ministry of public health (moph). The eu mdr 2017/745 came into effect in may 2021. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The two bodies regulating medical device registration in lebanon are the drug import and export department and. Access the. Medical Device Approval Lebanon.
From www.proximacro.com
510(k) or PMA Should Your Medical Device Receive FDA Clearance or FDA Medical Device Approval Lebanon All medical devices certified under the previous medical device directive (mdd) must. The ministry of public health regulates medical devices in lebanon. Access the list of regulatied products, classifications and approval. The medical device market in lebanon mostly relies on. The eu mdr 2017/745 came into effect in may 2021. All medical devices are regulated by the ministry of public. Medical Device Approval Lebanon.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Medical Device Approval Lebanon All medical devices are regulated by the ministry of public health (moph). Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The ministry of public health regulates medical devices in lebanon. The two bodies regulating medical device registration in lebanon are the drug import and export department and.. Medical Device Approval Lebanon.
From www.iqvia.com
FDA Publishes Approved List of AI/MLenabled Medical Devices IQVIA Medical Device Approval Lebanon All medical devices are regulated by the ministry of public health (moph). All medical devices certified under the previous medical device directive (mdd) must. The ministry of public health regulates medical devices in lebanon. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The medical device market in. Medical Device Approval Lebanon.
From www.vrogue.co
Fda Medical Device Approval Process vrogue.co Medical Device Approval Lebanon The medical device market in lebanon mostly relies on. The eu mdr 2017/745 came into effect in may 2021. Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. Lebanon. Medical Device Approval Lebanon.
From www.rarediseaseadvisor.com
RealWorld Data, Evidence Help Drive Rare Disease Drug and Device Approval Medical Device Approval Lebanon The two bodies regulating medical device registration in lebanon are the drug import and export department and. The ministry of public health regulates medical devices in lebanon. Access the list of regulatied products, classifications and approval. All medical devices are regulated by the ministry of public health (moph). The medical device market in lebanon mostly relies on. All medical devices. Medical Device Approval Lebanon.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Approval Lebanon The eu mdr 2017/745 came into effect in may 2021. Access the list of regulatied products, classifications and approval. The ministry of public health regulates medical devices in lebanon. All medical devices certified under the previous medical device directive (mdd) must. Lebanon is in a great location in the middle east, making it a good place for medical device companies. Medical Device Approval Lebanon.
From www.slideteam.net
Six Months Roadmap Of Medical Device Approval From FDA Regulatory Medical Device Approval Lebanon The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The ministry of public health regulates medical devices in lebanon. All medical devices are regulated by the ministry of public health (moph). Access the list of regulatied products, classifications and approval. The medical device market in lebanon mostly relies on. All. Medical Device Approval Lebanon.
From www.healthleadersmedia.com
CMS Previews Reboot of Medical Device Approval Pathways HealthLeaders Medical Device Approval Lebanon Access the list of regulatied products, classifications and approval. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. All medical devices certified under the previous medical device directive (mdd) must. All medical devices are regulated by the ministry of public health (moph). The ministry of health (moph), in. Medical Device Approval Lebanon.
From sterlingmedicaldevices.com
Strategies for Navigating Medical Device FDA and CE Approval Medical Device Approval Lebanon The ministry of public health regulates medical devices in lebanon. The eu mdr 2017/745 came into effect in may 2021. Access the list of regulatied products, classifications and approval. All medical devices are regulated by the ministry of public health (moph). Lebanon is in a great location in the middle east, making it a good place for medical device companies. Medical Device Approval Lebanon.
From aaos.org
An Overview of the FDA Approval Process for Devices Medical Device Approval Lebanon Access the list of regulatied products, classifications and approval. All medical devices certified under the previous medical device directive (mdd) must. The medical device market in lebanon mostly relies on. The ministry of public health regulates medical devices in lebanon. The two bodies regulating medical device registration in lebanon are the drug import and export department and. The eu mdr. Medical Device Approval Lebanon.
From www.youtube.com
The 5 most important steps to CE certification The EU medical device Medical Device Approval Lebanon The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The two bodies regulating medical device registration in lebanon are the drug import and export department and. Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The medical device. Medical Device Approval Lebanon.
From www.slideshare.net
US FDA medical device approval chart Emergo Medical Device Approval Lebanon Lebanon is in a great location in the middle east, making it a good place for medical device companies to grow. The medical device market in lebanon mostly relies on. The two bodies regulating medical device registration in lebanon are the drug import and export department and. All medical devices certified under the previous medical device directive (mdd) must. The. Medical Device Approval Lebanon.
From www.mokomedtech.com
How to Get FDA Approval for Medical Devices MokoMedtech Professional Medical Device Approval Lebanon The ministry of health (moph), in cooperation with the “agence française de sécurité du médicament et des produits de. The two bodies regulating medical device registration in lebanon are the drug import and export department and. Access the list of regulatied products, classifications and approval. The ministry of public health regulates medical devices in lebanon. The eu mdr 2017/745 came. Medical Device Approval Lebanon.
From www.slideshare.net
US FDA medical device approval chart Emergo Medical Device Approval Lebanon All medical devices certified under the previous medical device directive (mdd) must. All medical devices are regulated by the ministry of public health (moph). Access the list of regulatied products, classifications and approval. The two bodies regulating medical device registration in lebanon are the drug import and export department and. The ministry of health (moph), in cooperation with the “agence. Medical Device Approval Lebanon.