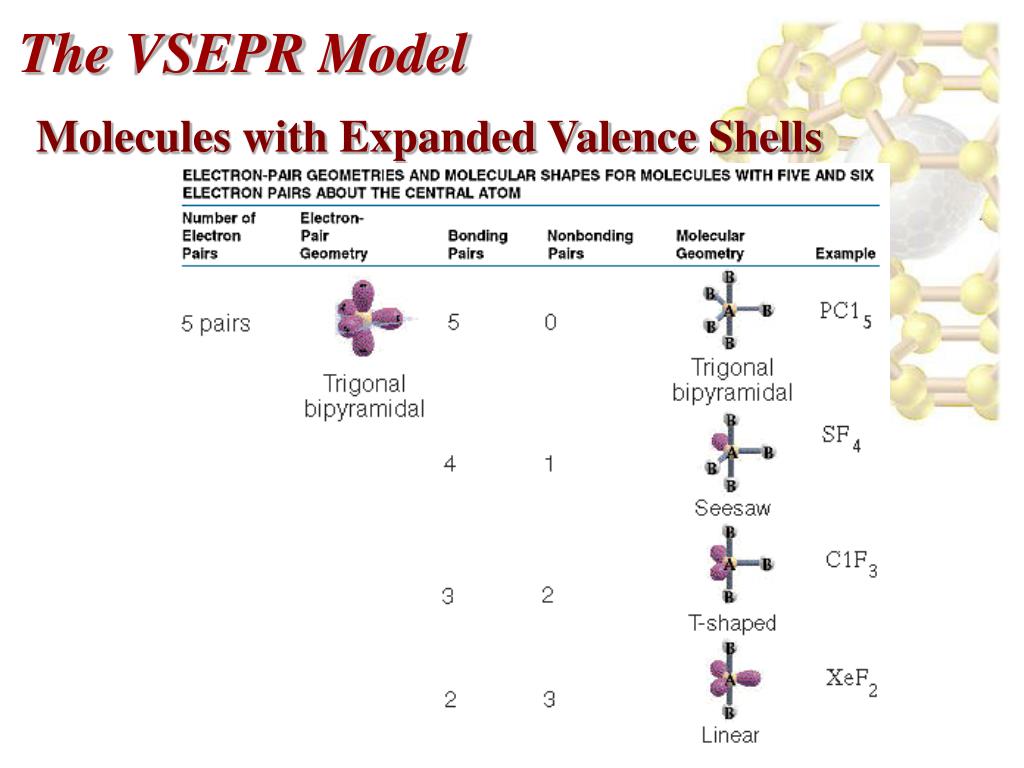
Expanded Valence Shell Molecules Definition . More common than incomplete octets are expanded octets where the central atom in a lewis. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Breakdown of the octet rule. Odd number of valence electrons. Write the chemical formulas and chemical names of covalent molecules that. Identify which elements can expand their valences when forming covalent molecules. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. This achieves a stable electron.
from www.slideserve.com
The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. This achieves a stable electron. Identify which elements can expand their valences when forming covalent molecules. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Breakdown of the octet rule. More common than incomplete octets are expanded octets where the central atom in a lewis. Odd number of valence electrons. Write the chemical formulas and chemical names of covalent molecules that.
PPT Molecular Shapes PowerPoint Presentation, free download ID6172622
Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. Write the chemical formulas and chemical names of covalent molecules that. Identify which elements can expand their valences when forming covalent molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. Odd number of valence electrons. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. This achieves a stable electron. Breakdown of the octet rule. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Identify which elements can expand their valences when forming covalent molecules. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition More common than incomplete octets are expanded octets where the central atom in a lewis. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. This achieves a stable electron. Odd number of valence electrons. Write the chemical formulas and chemical names of covalent. Expanded Valence Shell Molecules Definition.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Expanded Valence Shell Molecules Definition Identify which elements can expand their valences when forming covalent molecules. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Breakdown of the octet rule. Write the chemical formulas and chemical names of covalent molecules that. Odd number of valence electrons. In chemistry,. Expanded Valence Shell Molecules Definition.
From studylib.net
Expanded valence shells (extended octets) Expanded Valence Shell Molecules Definition The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. More common than incomplete octets are expanded octets where the central atom in a lewis. This achieves a stable electron. The octet rule applies well to atoms in the second row of the periodic. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition Identify which elements can expand their valences when forming covalent molecules. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. More common than incomplete octets are expanded octets where the central atom in a lewis. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition Write the chemical formulas and chemical names of covalent molecules that. This achieves a stable electron. Breakdown of the octet rule. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. More common than incomplete octets are expanded octets where the central atom in. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free Expanded Valence Shell Molecules Definition Write the chemical formulas and chemical names of covalent molecules that. Odd number of valence electrons. More common than incomplete octets are expanded octets where the central atom in a lewis. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. The octet rule. Expanded Valence Shell Molecules Definition.
From www.youtube.com
Grade12 Chapter1 Lewis Structures of Electron deficient and Expanded Expanded Valence Shell Molecules Definition In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Breakdown of the octet rule. More common than incomplete octets are expanded octets where the central atom in a lewis. This achieves a stable electron. The octet rule applies well to atoms in the second row of the periodic table, where. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Molecular Shapes PowerPoint Presentation, free download ID6172622 Expanded Valence Shell Molecules Definition Write the chemical formulas and chemical names of covalent molecules that. Breakdown of the octet rule. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. This achieves a stable electron. The octet rule is a chemistry rule of thumb that says that atoms combine in. Expanded Valence Shell Molecules Definition.
From ar.inspiredpencil.com
Valence Electron Structure Expanded Valence Shell Molecules Definition This achieves a stable electron. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Write the chemical formulas and chemical names of covalent molecules that. More common than incomplete octets are expanded octets where the central atom in a lewis. Breakdown of the. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Molecular Geometry PowerPoint Presentation ID2422169 Expanded Valence Shell Molecules Definition Identify which elements can expand their valences when forming covalent molecules. Odd number of valence electrons. Write the chemical formulas and chemical names of covalent molecules that. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Breakdown of the octet rule. This achieves a stable electron. The octet rule applies. Expanded Valence Shell Molecules Definition.
From slideplayer.com
Molecular Geometry & Bonding Theories ppt download Expanded Valence Shell Molecules Definition This achieves a stable electron. Breakdown of the octet rule. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. Identify which elements can expand their valences when forming covalent molecules. The octet rule is a chemistry rule of thumb that says that atoms combine in. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Lecture 24 Beyond the Octet PowerPoint Presentation, free Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. This achieves a stable electron. Odd number of valence electrons. The octet rule is a chemistry rule. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Breakdown of the octet rule. This achieves a stable electron. More common than incomplete octets. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Resonance Structures PowerPoint Presentation, free download ID Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. More common than incomplete octets are expanded octets where the central atom in a lewis. Odd number of valence electrons. The octet rule is a chemistry rule of thumb that says that atoms combine in a. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. This achieves a stable electron. Identify which elements can expand their valences when forming covalent molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. In chemistry, a. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition More common than incomplete octets are expanded octets where the central atom in a lewis. Identify which elements can expand their valences when forming covalent molecules. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Write the chemical formulas and chemical names of covalent molecules that. The octet rule applies. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Molecular Geometry and Bonding Theories (CH.9) PowerPoint Expanded Valence Shell Molecules Definition Odd number of valence electrons. This achieves a stable electron. Breakdown of the octet rule. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Identify which. Expanded Valence Shell Molecules Definition.
From www.numerade.com
SOLVEDWhich elements can form expanded valence shell molecules? Do the Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Write the chemical formulas and chemical names of covalent molecules that. More common than incomplete octets are. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Chapter 8 Concepts of Chemical Bonding (85 to 88) PowerPoint Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. Breakdown of the octet rule. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Write the chemical formulas and chemical names of covalent molecules that. More. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. Identify which elements can expand their valences when forming covalent molecules. Breakdown of the octet rule. This achieves a stable electron. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is. Expanded Valence Shell Molecules Definition.
From www.numerade.com
SOLVED To represent the right Lewis structure of one of these Expanded Valence Shell Molecules Definition Odd number of valence electrons. This achieves a stable electron. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Identify which elements can expand. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Chapter 9 Molecular Geometries and Bonding Theories PowerPoint Expanded Valence Shell Molecules Definition Identify which elements can expand their valences when forming covalent molecules. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Write the chemical formulas and chemical. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Exceptions to the Octet Rule PowerPoint Presentation, free Expanded Valence Shell Molecules Definition Identify which elements can expand their valences when forming covalent molecules. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. Odd number of valence electrons. Breakdown of the octet rule. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition This achieves a stable electron. More common than incomplete octets are expanded octets where the central atom in a lewis. Odd number of valence electrons. Breakdown of the octet rule. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. In chemistry, a hypervalent molecule (the. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition Write the chemical formulas and chemical names of covalent molecules that. Identify which elements can expand their valences when forming covalent molecules. Breakdown of the octet rule. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. In chemistry, a hypervalent molecule (the phenomenon is sometimes. Expanded Valence Shell Molecules Definition.
From www.youtube.com
VSEPR for Expanded Valence Shell YouTube Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Write the chemical formulas and chemical names of covalent molecules. Expanded Valence Shell Molecules Definition.
From www.youtube.com
Expanded Valence Shell Bonding YouTube Expanded Valence Shell Molecules Definition In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. This achieves a stable electron. Identify which elements can expand their valences when forming covalent molecules. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. More. Expanded Valence Shell Molecules Definition.
From slideplayer.com
Chapter 9 Chapter 9 Molecular Geometry and Bonding Theories. ppt download Expanded Valence Shell Molecules Definition In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. This achieves a stable electron. Breakdown of the octet rule. Odd number of valence electrons. More common. Expanded Valence Shell Molecules Definition.
From www.expii.com
Valence Electrons — Definition & Importance Expii Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. Breakdown of the octet rule. Odd number of valence electrons. Write the chemical formulas and chemical names of covalent molecules that. The octet rule is a chemistry rule of thumb that says that atoms combine in. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition Write the chemical formulas and chemical names of covalent molecules that. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Bonding and Molecular Structure Fundamental Concepts PowerPoint Expanded Valence Shell Molecules Definition This achieves a stable electron. Write the chemical formulas and chemical names of covalent molecules that. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. Odd number of valence electrons. More common than incomplete octets are expanded octets where the central atom in. Expanded Valence Shell Molecules Definition.
From slidetodoc.com
Molecular Geometry Bonding Theories l Molecules have shapes Expanded Valence Shell Molecules Definition The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. Odd number of valence electrons. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. This achieves a stable electron.. Expanded Valence Shell Molecules Definition.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Molecules Definition In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Breakdown of the octet rule. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. More common than incomplete octets are expanded octets where the central atom. Expanded Valence Shell Molecules Definition.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID4510494 Expanded Valence Shell Molecules Definition Identify which elements can expand their valences when forming covalent molecules. The octet rule applies well to atoms in the second row of the periodic table, where a full valence shell includes eight electrons with. The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence. Expanded Valence Shell Molecules Definition.